Page 143 - Materials Chemistry, Second Edition
P. 143
130 2 Solid-State Chemistry
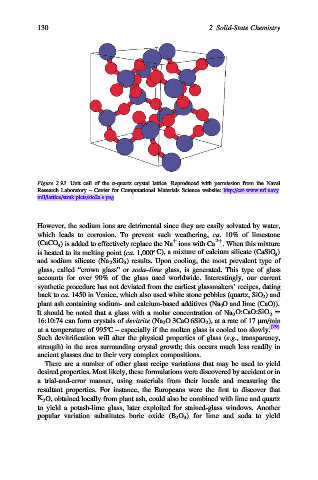
Figure 2.93. Unit cell of the a-quartz crystal lattice. Reproduced with permission from the Naval
Research Laboratory – Center for Computational Materials Science website: http://cst-www.nrl.navy.
mil/lattice/struk.picts/sio2a.s.png.
However, the sodium ions are detrimental since they are easily solvated by water,
which leads to corrosion. To prevent such weathering, ca. 10% of limestone
þ
2þ
(CaCO 3 ) is added to effectively replace the Na ions with Ca . When this mixture
is heated to its melting point (ca. 1,000 C), a mixture of calcium silicate (CaSiO 3 )
and sodium silicate (Na 2 SiO 3 ) results. Upon cooling, the most prevalent type of
glass, called “crown glass” or soda–lime glass, is generated. This type of glass
accounts for over 90% of the glass used worldwide. Interestingly, our current
synthetic procedure has not deviated from the earliest glassmakers’ recipes, dating
back to ca. 1450 in Venice, which also used white stone pebbles (quartz, SiO 2 ) and
plant ash containing sodium- and calcium-based additives (Na 2 O and lime (CaO)).
It should be noted that a glass with a molar concentration of Na 2 O:CaO:SiO 2 ¼
. .
16:10:74 can form crystals of devitrite (Na 2 O 3CaO 6SiO 2 ), at a rate of 17 mm/min
[79]
at a temperature of 995 C – especially if the molten glass is cooled too slowly.
Such devitrification will alter the physical properties of glass (e.g., transparency,
strength) in the area surrounding crystal growth; this occurs much less readily in
ancient glasses due to their very complex compositions.
There are a number of other glass recipe variations that may be used to yield
desired properties. Most likely, these formulations were discovered by accident or in
a trial-and-error manner, using materials from their locale and measuring the
resultant properties. For instance, the Europeans were the first to discover that
K 2 O, obtained locally from plant ash, could also be combined with lime and quartz
to yield a potash-lime glass, later exploited for stained-glass windows. Another
popular variation substitutes boric oxide (B 2 O 3 ) for lime and soda to yield