Page 82 - Materials Chemistry, Second Edition
P. 82
69
2.3. The Crystalline State
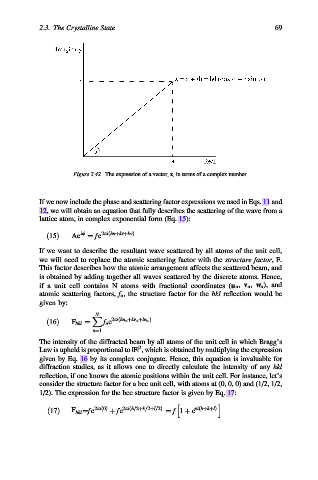
Figure 2.42. The expression of a vector, z, in terms of a complex number.
If we now include the phase and scattering factor expressions we used in Eqs. 11 and
12, we will obtain an equation that fully describes the scattering of the wave from a
lattice atom, in complex exponential form (Eq. 15):
if
ð15Þ Ae ¼ fe 2piðhuþkvþlwÞ
If we want to describe the resultant wave scattered by all atoms of the unit cell,
we will need to replace the atomic scattering factor with the structure factor,F.
This factor describes how the atomic arrangement affects the scattered beam, and
is obtained by adding together all waves scattered by the discrete atoms. Hence,
if a unit cell contains N atoms with fractional coordinates (u n , v n , w n ), and
atomic scattering factors, f n , the structure factor for the hkl reflection would be
given by:
N
X
ð16Þ F hkl ¼ f n e 2piðhu n þkv n þlw n Þ
n¼1
The intensity of the diffracted beam by all atoms of the unit cell in which Bragg’s
2
Law is upheld is proportional to |F| , which is obtained by multiplying the expression
given by Eq. 16 by its complex conjugate. Hence, this equation is invaluable for
diffraction studies, as it allows one to directly calculate the intensity of any hkl
reflection, if one knows the atomic positions within the unit cell. For instance, let’s
consider the structure factor for a bcc unit cell, with atoms at (0, 0, 0) and (1/2, 1/2,
1/2). The expression for the bcc structure factor is given by Eq. 17:
h i
ð17Þ F hkl ¼fe 2pið0Þ þ fe 2piðh=2þk=2þl=2Þ ¼ f 1 þ e piðhþkþlÞ