Page 90 - Materials Chemistry, Second Edition
P. 90
77
2.3. The Crystalline State
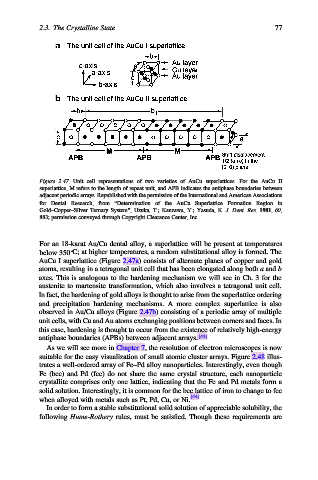
Figure 2.47. Unit cell representations of two varieties of AuCu superlattices. For the AuCu II
superlattice, M refers to the length of repeat unit, and APB indicates the antiphase boundaries between
adjacent periodic arrays. Republished with the permission of the International and American Associations
for Dental Research, from “Determination of the AuCu Superlattice Formation Region in
Gold–Copper–Silver Ternary System”, Uzuka, T.; Kanzawa, Y.; Yasuda, K. J. Dent. Res. 1981, 60,
883; permission conveyed through Copyright Clearance Center, Inc.
For an 18-karat Au/Cu dental alloy, a superlattice will be present at temperatures
below 350 C; at higher temperatures, a random substitutional alloy is formed. The
AuCu I superlattice (Figure 2.47a) consists of alternate planes of copper and gold
atoms, resulting in a tetragonal unit cell that has been elongated along both a and b
axes. This is analogous to the hardening mechanism we will see in Ch. 3 for the
austenite to martensite transformation, which also involves a tetragonal unit cell.
In fact, the hardening of gold alloys is thought to arise from the superlattice ordering
and precipitation hardening mechanisms. A more complex superlattice is also
observed in Au/Cu alloys (Figure 2.47b) consisting of a periodic array of multiple
unit cells, with Cu and Au atoms exchanging positions between corners and faces. In
this case, hardening is thought to occur from the existence of relatively high-energy
antiphase boundaries (APBs) between adjacent arrays. [43]
As we will see more in Chapter 7, the resolution of electron microscopes is now
suitable for the easy visualization of small atomic cluster arrays. Figure 2.48 illus-
trates a well-ordered array of Fe–Pd alloy nanoparticles. Interestingly, even though
Fe (bcc) and Pd (fcc) do not share the same crystal structure, each nanoparticle
crystallite comprises only one lattice, indicating that the Fe and Pd metals form a
solid solution. Interestingly, it is common for the bcc lattice of iron to change to fcc
when alloyed with metals such as Pt, Pd, Cu, or Ni. [44]
In order to form a stable substitutional solid solution of appreciable solubility, the
following Hume-Rothery rules, must be satisfied. Though these requirements are