Page 91 - Materials Chemistry, Second Edition
P. 91
78 2 Solid-State Chemistry
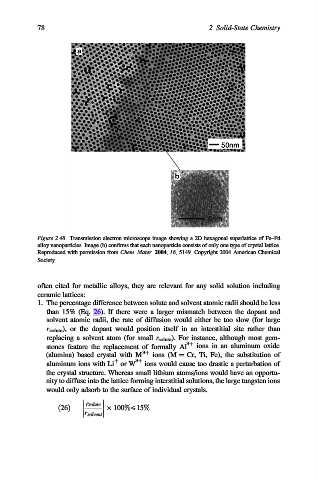
Figure 2.48. Transmission electron microscope image showing a 2D hexagonal superlattice of Fe–Pd
alloy nanoparticles. Image (b) confirms that each nanoparticle consists of only one type of crystal lattice.
Reproduced with permission from Chem. Mater. 2004, 16, 5149. Copyright 2004 American Chemical
Society.
often cited for metallic alloys, they are relevant for any solid solution including
ceramic lattices:
1. The percentage difference between solute and solvent atomic radii should be less
than 15% (Eq. 26). If there were a larger mismatch between the dopant and
solvent atomic radii, the rate of diffusion would either be too slow (for large
r solute ), or the dopant would position itself in an interstitial site rather than
replacing a solvent atom (for small r solute ). For instance, although most gem-
stones feature the replacement of formally Al 3þ ions in an aluminum oxide
(alumina) based crystal with M 3þ ions (M ¼ Cr, Ti, Fe), the substitution of
þ
aluminum ions with Li or W 3þ ions would cause too drastic a perturbation of
the crystal structure. Whereas small lithium atoms/ions would have an opportu-
nity to diffuse into the lattice forming interstitial solutions, the large tungsten ions
would only adsorb to the surface of individual crystals.
r solute
ð26Þ 100%b15%
r solvent