Page 89 - Materials Chemistry, Second Edition
P. 89
76 2 Solid-State Chemistry
There are four main classifications of crystalline imperfections that exist in
crystalline solids:
(a) Point defects – interstitial/substitutional dopants, Schottky/Frenkel defects,
voids (vacancies)
(b) Linear defects – edge and screw dislocations
(c) Planar defects – grain boundaries, surfaces
(d) Bulk defects – pores, cracks
Of these four types, point/linear defects may only be observed at the atomic level,
requiring sophisticated electron microscopy. In contrast, planar defects are often
visible using a light microscope, and bulk defects are easily observed by the naked eye.
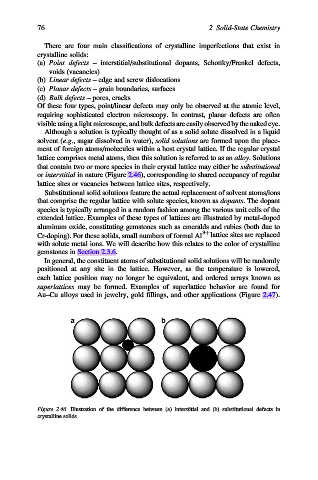
Although a solution is typically thought of as a solid solute dissolved in a liquid
solvent (e.g., sugar dissolved in water), solid solutions are formed upon the place-
ment of foreign atoms/molecules within a host crystal lattice. If the regular crystal
lattice comprises metal atoms, then this solution is referred to as an alloy. Solutions
that contain two or more species in their crystal lattice may either be substitutional
or interstitial in nature (Figure 2.46), corresponding to shared occupancy of regular
lattice sites or vacancies between lattice sites, respectively.
Substitutional solid solutions feature the actual replacement of solvent atoms/ions
that comprise the regular lattice with solute species, known as dopants. The dopant
species is typically arranged in a random fashion among the various unit cells of the
extended lattice. Examples of these types of lattices are illustrated by metal-doped
aluminum oxide, constituting gemstones such as emeralds and rubies (both due to
Cr-doping). For these solids, small numbers of formal Al 3þ lattice sites are replaced
with solute metal ions. We will describe how this relates to the color of crystalline
gemstones in Section 2.3.6.
In general, the constituent atoms of substitutional solid solutions will be randomly
positioned at any site in the lattice. However, as the temperature is lowered,
each lattice position may no longer be equivalent, and ordered arrays known as
superlattices may be formed. Examples of superlattice behavior are found for
Au–Cu alloys used in jewelry, gold fillings, and other applications (Figure 2.47).
Figure 2.46. Illustration of the difference between (a) interstitial and (b) substitutional defects in
crystalline solids.