Page 453 - Mechanical Engineers' Handbook (Volume 4)
P. 453
442 Refrigeration
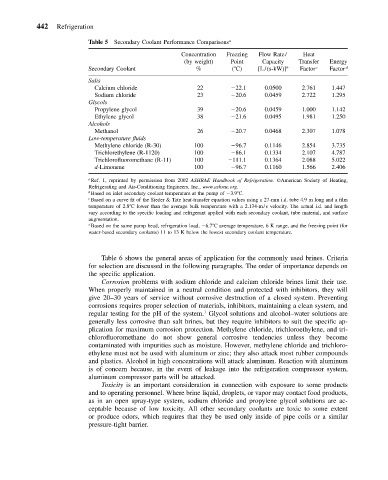
Table 5 Secondary Coolant Performance Comparisons a
Concentration Freezing Flow Rate/ Heat
(by weight) Point Capacity Transfer Energy
Secondary Coolant % ( C) [L/(s-kW)] b Factor c Factor d
Salts
Calcium chloride 22 22.1 0.0500 2.761 1.447
Sodium chloride 23 20.6 0.0459 2.722 1.295
Glycols
Propylene glycol 39 20.6 0.0459 1.000 1.142
Ethylene glycol 38 21.6 0.0495 1.981 1.250
Alcohols
Methanol 26 20.7 0.0468 2.307 1.078
Low-temperature fluids
Methylene chloride (R-30) 100 96.7 0.1146 2.854 3.735
Trichlorethylene (R-1120) 100 86.1 0.1334 2.107 4.787
Trichlorofluoromethane (R-11) 100 111.1 0.1364 2.088 5.022
d-Limonene 100 96.7 0.1160 1.566 2.406
a Ref. 1, reprinted by permission from 2002 ASHRAE Handbook of Refrigeration. American Society of Heating,
Refrigerating and Air-Conditioning Engineers, Inc., www.ashrae.org.
b Based on inlet secondary coolant temperature at the pump of 3.9 C.
c Based on a curve fit of the Sieder & Tate heat-transfer equation values using a 27-mm i.d. tube 4.9 m long and a film
temperature of 2.8 C lower than the average bulk temperature with a 2.134-m/s velocity. The actual i.d. and length
vary according to the specific loading and refrigerant applied with each secondary coolant, tube material, and surface
augmentation.
d Based on the same pump head, refrigeration load, 6.7 C average temperature, 6 K range, and the freezing point (for
water-based secondary coolants) 11 to 13 K below the lowest secondary coolant temperature.
Table 6 shows the general areas of application for the commonly used brines. Criteria
for selection are discussed in the following paragraphs. The order of importance depends on
the specific application.
Corrosion problems with sodium chloride and calcium chloride brines limit their use.
When properly maintained in a neutral condition and protected with inhibitors, they will
give 20–30 years of service without corrosive destruction of a closed system. Preventing
corrosions requires proper selection of materials, inhibitors, maintaining a clean system, and
regular testing for the pH of the system. Glycol solutions and alcohol–water solutions are
1
generally less corrosive than salt brines, but they require inhibitors to suit the specific ap-
plication for maximum corrosion protection. Methylene chloride, trichloroethylene, and tri-
chlorofluoromethane do not show general corrosive tendencies unless they become
contaminated with impurities such as moisture. However, methylene chloride and trichloro-
ethylene must not be used with aluminum or zinc; they also attack most rubber compounds
and plastics. Alcohol in high concentrations will attack aluminum. Reaction with aluminum
is of concern because, in the event of leakage into the refrigeration compressor system,
aluminum compressor parts will be attacked.
Toxicity is an important consideration in connection with exposure to some products
and to operating personnel. Where brine liquid, droplets, or vapor may contact food products,
as in an open spray-type system, sodium chloride and propylene glycol solutions are ac-
ceptable because of low toxicity. All other secondary coolants are toxic to some extent
or produce odors, which requires that they be used only inside of pipe coils or a similar
pressure-tight barrier.