Page 454 - Mechanical Engineers' Handbook (Volume 4)
P. 454
6 Indirect Refrigeration 443
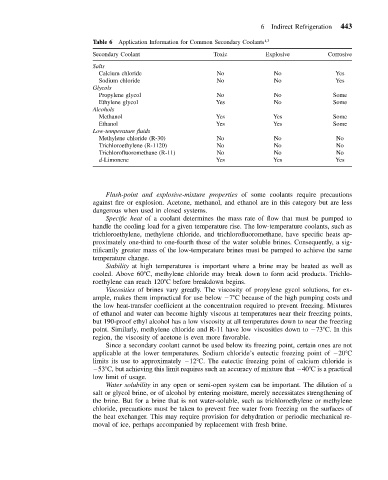
Table 6 Application Information for Common Secondary Coolants 1,3
Secondary Coolant Toxic Explosive Corrosive
Salts
Calcium chloride No No Yes
Sodium chloride No No Yes
Glycols
Propylene glycol No No Some
Ethylene glycol Yes No Some
Alcohols
Methanol Yes Yes Some
Ethanol Yes Yes Some
Low-temperature fluids
Methylene chloride (R-30) No No No
Trichloroethylene (R-1120) No No No
Trichlorofluoromethane (R-11) No No No
d-Limonene Yes Yes Yes
Flash-point and explosive-mixture properties of some coolants require precautions
against fire or explosion. Acetone, methanol, and ethanol are in this category but are less
dangerous when used in closed systems.
Specific heat of a coolant determines the mass rate of flow that must be pumped to
handle the cooling load for a given temperature rise. The low-temperature coolants, such as
trichloroethylene, methylene chloride, and trichlorofluoromethane, have specific heats ap-
proximately one-third to one-fourth those of the water soluble brines. Consequently, a sig-
nificantly greater mass of the low-temperature brines must be pumped to achieve the same
temperature change.
Stability at high temperatures is important where a brine may be heated as well as
cooled. Above 60 C, methylene chloride may break down to form acid products. Trichlo-
roethylene can reach 120 C before breakdown begins.
Viscosities of brines vary greatly. The viscosity of propylene gycol solutions, for ex-
ample, makes them impractical for use below 7 C because of the high pumping costs and
the low heat-transfer coefficient at the concentration required to prevent freezing. Mixtures
of ethanol and water can become highly viscous at temperatures near their freezing points,
but 190-proof ethyl alcohol has a low viscosity at all temperatures down to near the freezing
point. Similarly, methylene chloride and R-11 have low viscosities down to 73 C. In this
region, the viscosity of acetone is even more favorable.
Since a secondary coolant cannot be used below its freezing point, certain ones are not
applicable at the lower temperatures. Sodium chloride’s eutectic freezing point of 20 C
limits its use to approximately 12 C. The eutectic freezing point of calcium chloride is
53 C, but achieving this limit requires such an accuracy of mixture that 40 C is a practical
low limit of usage.
Water solubility in any open or semi-open system can be important. The dilution of a
salt or glycol brine, or of alcohol by entering moisture, merely necessitates strengthening of
the brine. But for a brine that is not water-soluble, such as trichloroethylene or methylene
chloride, precautions must be taken to prevent free water from freezing on the surfaces of
the heat exchanger. This may require provision for dehydration or periodic mechanical re-
moval of ice, perhaps accompanied by replacement with fresh brine.