Page 477 - Mechanical Engineers' Handbook (Volume 4)
P. 477
466 Cryogenic Systems
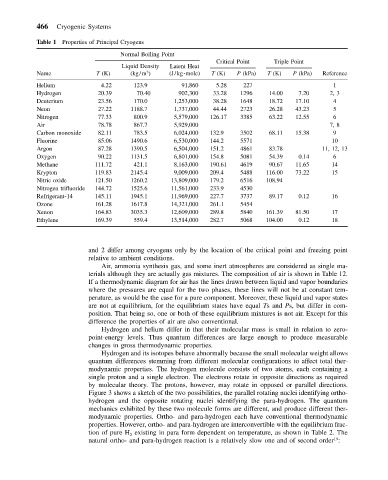
Table 1 Properties of Principal Cryogens
Normal Boiling Point
Critical Point Triple Point
Liquid Density Latent Heat
3
Name T (K) (kg/m ) (J/kg mole) T (K) P (kPa) T (K) P (kPa) Reference
Helium 4.22 123.9 91,860 5.28 227 1
Hydrogen 20.39 70.40 902,300 33.28 1296 14.00 7.20 2, 3
Deuterium 23.56 170.0 1,253,000 38.28 1648 18.72 17.10 4
Neon 27.22 1188.7 1,737,000 44.44 2723 26.28 43.23 5
Nitrogen 77.33 800.9 5,579,000 126.17 3385 63.22 12.55 6
Air 78.78 867.7 5,929,000 7, 8
Carbon monoxide 82.11 783.5 6,024,000 132.9 3502 68.11 15.38 9
Fluorine 85.06 1490.6 6,530,000 144.2 5571 10
Argon 87.28 1390.5 6,504,000 151.2 4861 83.78 11, 12, 13
Oxygen 90.22 1131.5 6,801,000 154.8 5081 54.39 0.14 6
Methane 111.72 421.1 8,163,000 190.61 4619 90.67 11.65 14
Krypton 119.83 2145.4 9,009,000 209.4 5488 116.00 73.22 15
Nitric oxide 121.50 1260.2 13,809,000 179.2 6516 108.94
Nitrogen trifluoride 144.72 1525.6 11,561,000 233.9 4530
Refrigerant-14 145.11 1945.1 11,969,000 227.7 3737 89.17 0.12 16
Ozone 161.28 1617.8 14,321,000 261.1 5454
Xenon 164.83 3035.3 12,609,000 289.8 5840 161.39 81.50 17
Ethylene 169.39 559.4 13,514,000 282.7 5068 104.00 0.12 18
and 2 differ among cryogens only by the location of the critical point and freezing point
relative to ambient conditions.
Air, ammonia synthesis gas, and some inert atmospheres are considered as single ma-
terials although they are actually gas mixtures. The composition of air is shown in Table 12.
If a thermodynamic diagram for air has the lines drawn between liquid and vapor boundaries
where the pressures are equal for the two phases, these lines will not be at constant tem-
perature, as would be the case for a pure component. Moreover, these liquid and vapor states
are not at equilibrium, for the equilibrium states have equal Ts and Ps, but differ in com-
position. That being so, one or both of these equilibrium mixtures is not air. Except for this
difference the properties of air are also conventional.
Hydrogen and helium differ in that their molecular mass is small in relation to zero-
point-energy levels. Thus quantum differences are large enough to produce measurable
changes in gross thermodynamic properties.
Hydrogen and its isotopes behave abnormally because the small molecular weight allows
quantum differences stemming from different molecular configurations to affect total ther-
modynamic properties. The hydrogen molecule consists of two atoms, each containing a
single proton and a single electron. The electrons rotate in opposite directions as required
by molecular theory. The protons, however, may rotate in opposed or parallel directions.
Figure 3 shows a sketch of the two possibilities, the parallel rotating nuclei identifying ortho-
hydrogen and the opposite rotating nuclei identifying the para-hydrogen. The quantum
mechanics exhibited by these two molecule forms are different, and produce different ther-
modynamic properties. Ortho- and para-hydrogen each have conventional thermodynamic
properties. However, ortho- and para-hydrogen are interconvertible with the equilibrium frac-
tion of pure H existing in para form dependent on temperature, as shown in Table 2. The
2
19
natural ortho- and para-hydrogen reaction is a relatively slow one and of second order :