Page 478 - Mechanical Engineers' Handbook (Volume 4)
P. 478
1 Cryogenics and Cryofluid Properties 467
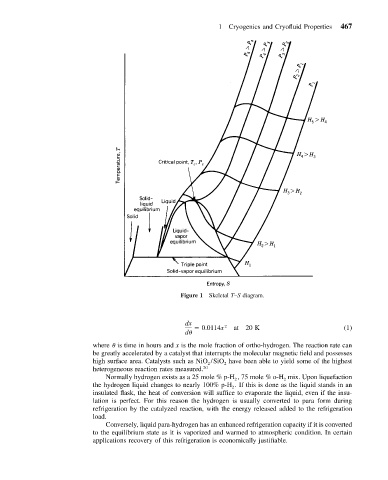
Figure 1 Skeletal T–S diagram.
dx
0.0114x 2 at 20 K (1)
d
where is time in hours and x is the mole fraction of ortho-hydrogen. The reaction rate can
be greatly accelerated by a catalyst that interrupts the molecular magnetic field and possesses
high surface area. Catalysts such as NiO /SiO have been able to yield some of the highest
2
2
heterogeneous reaction rates measured. 20
Normally hydrogen exists as a 25 mole % p-H , 75 mole % o-H mix. Upon liquefaction
2
2
the hydrogen liquid changes to nearly 100% p-H . If this is done as the liquid stands in an
2
insulated flask, the heat of conversion will suffice to evaporate the liquid, even if the insu-
lation is perfect. For this reason the hydrogen is usually converted to para form during
refrigeration by the catalyzed reaction, with the energy released added to the refrigeration
load.
Conversely, liquid para-hydrogen has an enhanced refrigeration capacity if it is converted
to the equilibrium state as it is vaporized and warmed to atmospheric condition. In certain
applications recovery of this refrigeration is economically justifiable.