Page 16 - Mechanism and Theory in Organic Chemistry
P. 16
Models of Chemical Bonding 5
There is a class of structures, however, for which the properties are not those
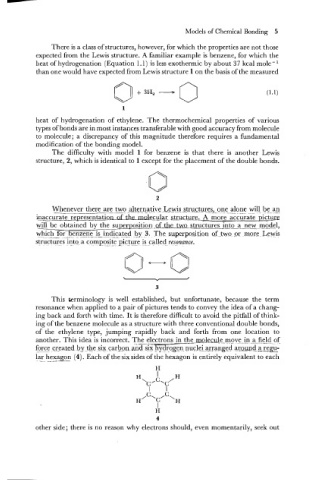
expected from the Lewis structure. A familiar example is benzene, for which the
heat of hydrogenation (Equation 1.1) is less exothermic by about 37 kcal mole -
than one would have expected from Lewis structure 1 on the basis of the measured
heat of hydrogenation of ethylene. The thermochemical properties of various
types of bonds are in most instances transferable with good accuracy from molecule
to molecule; a discrepancy of this magnitude therefore requires a fundamental
modification of the bonding model.
The difficulty with model 1 for benzene is that there is another Lewis
structure, 2, which is identical to 1 except for the placement of the double bonds.
Whenever there are two-ake_r-n_ativeLxwis _s_tructuyg_s,_one -- alone - will - be
inaccurate representation of th~de_cu_lar g~uct_ur_e, A more accurate picture
will be obtained by the superp~s~i~n~of the two structures into a new-model,
which --- for -. benzene is -- indicated by 3. The superposition of two or more Lewis
--
----
-
-
structures into a composite picture is called resonance.
-
This terminology is well established, but unfortunate, because the term
resonance when applied to a pair of pictures tends to convey the idea of a chang-
ing back and forth with time. It is therefore difficult to avoid the pitfall of think-
ing of the benzene molecule as a structure with three conventional double bonds,
of the ethylene type, jumping rapidly back and forth from one location to
another. This idea is incorrect. The electrons in the~molecule~m_ove in-a field of
<--
force - creakd by the six carbon and six hydrpgen nuclei arranged arou_nd_a_sw-
-
--
lar hexapn (4). Each of the six sides of the hexagon is entirely equivalent to each
----
other side; there is no reason why electrons should, even momentarily, seek out