Page 17 - Mechanism and Theory in Organic Chemistry
P. 17
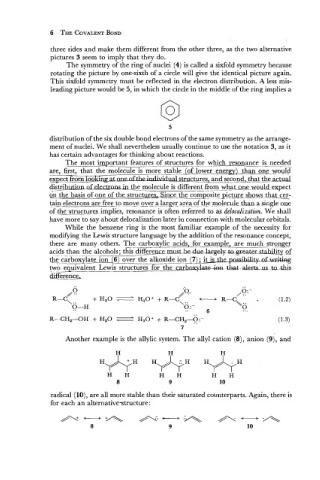
three sides and make them different from the other three, as the two alternative
pictures 3 seem to imply that they do.
The symmetry of the ring of nuclei (4) is called a sixfold symmetry because
rotating the picture by one-sixth of a circle will give the identical picture again.
This sixfold symmetry must be reflected in the electron distribution. A less mis-
leading picture would be 5, in which the circle in the middle of the ring implies a
distribution of the six double bond electrons of the same symmetry as the arrange-
ment of nuclei. We shall nevertheless usually continue to use the notation 3, as it
has certain advantages for thinking about reactions.
The most important features of structures for which resonance is needed
are, first, that the -f . .. lower energy) than on3 would
expect from looki~ structures, and second, that the actual
the-
of
distribution of ~mIISi~eem~oI_ecule is different --- -- frqm whhat*r?_e would expect
on the basis of one of the structures. Since the composite picture shows that cer-
tain electrons are free to move alarger area of the molecule than a single one
of the structures implies, resonanse is often referred to as delocalization. We shall
have more to say about delocalization later in connection with molecular orbitals.
While the benzene ring is the most familiar example of the necessity for
modifying the Lewis structure language by the addition of the resonance concept,
there are many others. The carboy& acids, for example, are much --- stron~
of
acids than the alcohols; t~s~ff~em.musbedudar&--tgsreate~stabili&
the k&b6k$iii id6kver the alkoxide ion17) ; it is the pwkd
-
' -Q=f-
- --.-- -
two equivalent Lewis structures for the carhax~ien.-~b;bt-..al91%.u..t0~
&fference,
Another example is the allylic system. The ally1 cation (8), anion (9), and
radical (lo), are all more stable than their saturated counterparts. Again, there is
for each an alternativestructure :