Page 18 - Mechanism and Theory in Organic Chemistry
P. 18
Models of Chemical Bonding 7
In all the examples we have considered so far, the alternative structures
have been equivalent. This will not always be the case, as the following examples
illustrate :
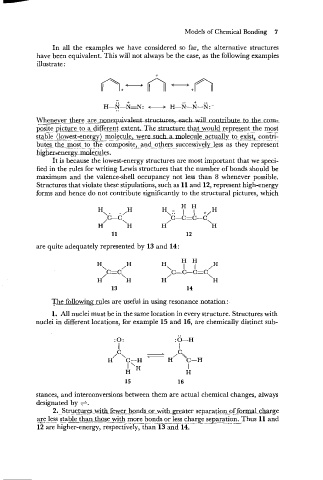
Whenever there3 n o n e q u i v a Q to contribute^^
posite_pjct.ire g.a.different__extent. The.stru_ctu_re~tbbatw~~~d~epre~~ttt
most
the
stable Ilowest-energy ) molecule - w~..ucL.m._1_ecule ._a.ctualk. foe xi st,^ .conzi-
.
butes_the-m.~st_to_ the composite,~. an6.others...successively .-less as they represent
hi&ereecules.
It is because the lowest-energy structures are most important that we speci-
fied in the rules for writing Lewis structures that the number of bonds should be
maximum and the valence-shell occupancy not less than 8 whenever possible.
Structures that violate these stipulations, such as 11 and 12, represent high-energy
forms and hence do not contribute significantly to the structural pictures, which
are quite adequately represented by 13 and 14:
The followinp; rules are useful in using resonance notatinn:
1. All nuclei must be in the same location in every structure. Structures with
nuclei in different locations, for example 15 and 16, are chemically distinct sub-
stances, and interconversions between them are actual chemical changes, always
designated by +.
2.. sStr_uc!ur~_w_ithfewer_ bon_d_s_so_ro_rw&hgeatte~I:~eparati.o-~~.of for~al-&a_rge
with
more
are ---- bonds or less charge sparation. Thus 11 and
stable
less
those
than
-
-- .- -- -
12 are higher-energy, respectively, than 13 and 14.