Page 22 - Mechanism and Theory in Organic Chemistry
P. 22
Molecular Orbitals 11
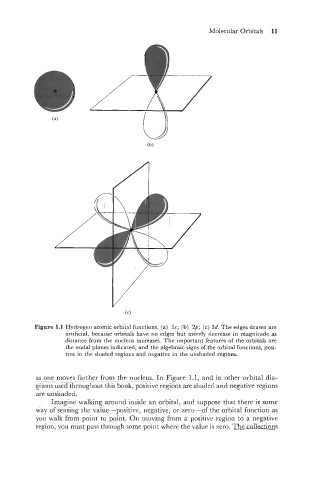
Figure 1.1 Hydrogen atomic orbital functions. (a) Is; (b) 2p; (c) 3d. The edges drawn are
artificial, because orbitals have no edges but merely decrease in magnitude as
distance from the nucleus increases. The important features of the orbitals are
the nodal planes indicated, and the algebraic signs of the orbital functions, posi-
tive in the shaded regions and negative in the unshaded regions.
as one moves farther from the nucleus. In Figure 1.1, and in other orbital dia-
-
grams used throughout this book, positive regions are shaded and negative regions
are unshaded.
Imagine walking around inside an orbital, and suppose that there is some
way of sensing the value-positive, negative, or zero-of the orbital function as
you walk from point to point. On moving from a positive region to a negative
region, you must pass through some point where the value is zero. T-ctions