Page 25 - Mechanism and Theory in Organic Chemistry
P. 25
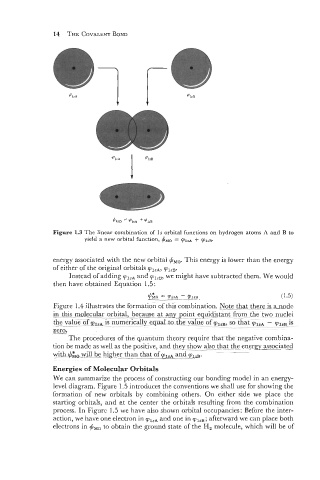
Figure 1.3 The linear combination of 1s orbital functions on hydrogen atoms A and B to
yield a new orbital function, I,!JMO = q~~~~ + vlP~.
#,,.
energy associated with the new orbital
This energy is lower than the energy
of either of the original orbitals ?,,A, q,,,.
Instead of adding y,,, and q~,,,, we might have subtracted them. We would
then have obtained Equation 1.5:
( l.5)
I,!J$o = VI~A - ~IISB
Figure 1.4 illustrates the formation of this combination. Note that~he~is
a&e
-
from-the two nuclei
inthi.s_mol.ec&ar orbital, because at-agpoint equidistant .
+
-
so that v,,, - -yls,~-is
thcl~ ofp,.is
numerically equal to the value of -
zero.
-
-
The procedures of the quantum theory require that the negative combina-
tion be made as well as the positive, and they show also that the energy associated
---
with #.&dl-bc higher --a than that of FICA and ?La.
Energies of Molecular Orbitals
We can summarize the process of constructing our bonding model in an energy-
level diagram. Figure 1.5 introduces the conventions we shall use for showing the
formation of new orbitals by combining others. On either side we place the
starting orbitals, and at the center the orbitals resulting from the combination
process. In Figure 1.5 we have also shown orbital occupancies: Before the inter-
action, we have one electron in ?,,, and one in ?,,, ; afterward we can place both
electrons in $,, to obtain the ground state of the H, molecule, which will be of