Page 29 - Mechanism and Theory in Organic Chemistry
P. 29
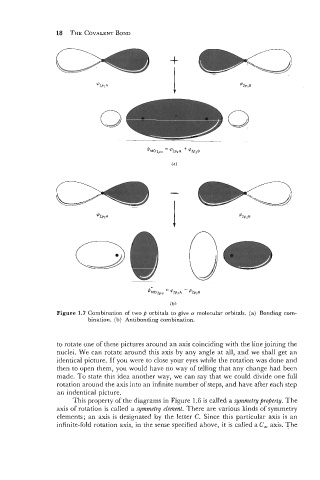
Figure 1.7 Combination of two p orbitals to give o molecular orbitals. (a) Bonding com-
bination. (b) Antibonding combination.
to rotate one of these pictures around an axis coinciding with the line joining the
nuclei. We can rotate around this axis by any angle at all, and we shall get an
identical picture. If you were to close your eyes while the rotation was done and
then to open them, you would have no way of telling that any change had been
made. To state this idea another way, we can say that we could divide one full
rotation around the axis into an infinite number ofsteps, and have after each step
an indentical picture.
This property of the diagrams in Figure 1.6 is called a symmetry property. The
axis of rotation is called a symmetry element. There are various kinds of symmetry
elements; an axis is designated by the letter C. Since this particular axis is an
infinite-fold rotation axis, in the sense specified above, it is called a C, axis. The