Page 27 - Mechanism and Theory in Organic Chemistry
P. 27
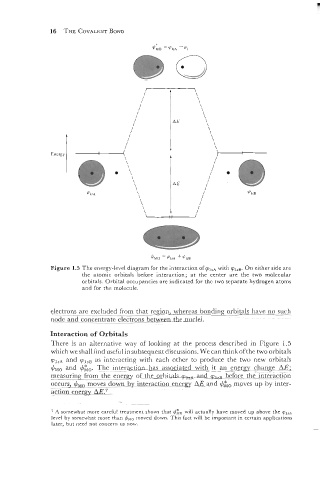
Figure 1.5 The energy-level diagram for the interaction ofvlSA with v,,,. On either side are
the atomic orbitals before interaction; at the center are the two molecular
orbitals. Orbital occupancies are indicated for the two separate hydrogen atoms
and for the molecule.
-
electrons --. - are excluded from that re~ion, whereas bonding orbitals have no such
node and concentrate electrco_ns-betlyem_thed.
Interaction of Orbitals
There is an alternative way of looking at the process described in Figure 1.5
which we shall find useful in subsequent discussions. We can think ofthe two orbitals
q?ls~ and v,,, as interacting with each other to produce the two new orbitals
+,, and The interacti~ has associated with it an energy chanze -AE;
measuring from the enerpv of the orbitals- qll~ before the interaction
OCCU~S~-$~~ moves-do-wn by interaction energy AE and I/&, moves up by inter-
-
actio~energy AE.7
A somewhat more careful treatment shows that +hco will actually have moved up above the a,,,
level by somewhat more than t,h,o moved down. This fact will be important in certain applications
later, but necd not concern us now.