Page 30 - Mechanism and Theory in Organic Chemistry
P. 30
Molecular Orbitals 19
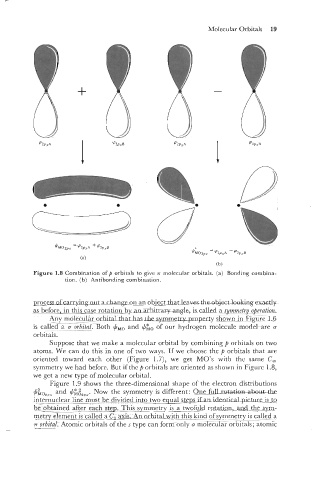
Figure 1.8 Combination of p orbitals to give .rr molecular orbitals. (a) Bonding combina-
tion. (b) Antibonding combination.
exa&€ly
on an obiect~b~e~b~thelci~
process of can $ngat.-
symmetry oter-ion,.
as before. in this case rotation by anarhitrar-yangle, .is&-d-a
Any molecular-WAuthas the symmetry prqerty shown inhasFiguce..l,_6
-___I
is called a - o orbztal. Both (G,,
and (GCo of our hydrogen molecule model are a
--
orbitals.
Suppose that we make a molecular orbital by combining fi orbitals on two
atoms. We can do this in one of two ways. If we choose thc p orbitals that are -
oriented toward each other (Figure 1.7), we get MO's with the same C,
symmetry we had before. But if the p orbitals are oriented as shown in Figure 1.8,
we get a new type of molecular orbital.
Figure 1.9 shows the three-dimensional shape of the electron distributions
y!~&~~~~ I,!I&~,~~. Now the symmetry is different: One full-he
and
into
internuclear line rnu~be~diy.~ two equal steps if.anidentical~ei~
b.6 obtained after each step. This symmetry is a twofold rol-he sym-
maelement is called a 6, axis. An mhitaly&h this kind ofz~metry is called2
-rr orbital. Atomic orbitals of the s type can form only o molecular orbitals; atomic