Page 35 - Mechanism and Theory in Organic Chemistry
P. 35
The advantage we gain by making hybrid orbitals is that we now have four
new atomic orbitals on carbon, each one oriented directly toward one of the
hydrogen atoms. Each hybrid will have a large overlap and therefore a large
interaction with one, but only one, hydrogen. Our complicated original problem,
in which each hydrogen 1s orbital had to interact with all four carbon atomic
orbitals, is now replaced by four separate but simple problems.
MOs from Hybrid Orbitals
We can now proceed to make molecular orbitals in the same way we did for H,.
Figure 1.12 shows the form of the bonding molecular orbital obtained from y,,,
and xSpsl; there will also be an antibonding combination which has a node be-
tween the atoms. The energy changes (Figure 1.13) follow the pattern we found
in H,. The only difference is that now the two interacting atomic orbitals are not
the same and have different energies. The energy difference in this instance is not
large, and makes no fundamental change in our model. We shall return to this
point in Chapter 10. Note that our new molecular orbitals have infinite-fold
axis, and so are cr orbitals.
symmetry about the C-H
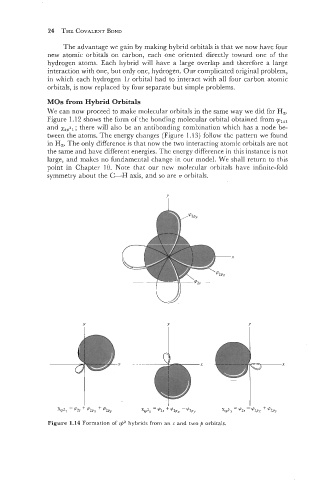
Figure 1.14 Formation of s,h2 hybrids from an s and two p orbitals.