Page 40 - Mechanism and Theory in Organic Chemistry
P. 40
Aromaticity 29
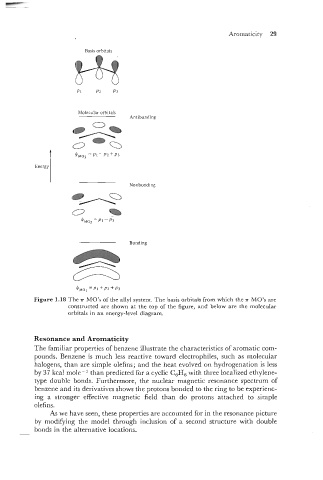
Basis orbitals
Molecular orbitals
Antibonding
Energy
Bonding
Figure 1.18 The .rr MO's of the ally1 system. The basis orbitals from which the rr MO's are
constructed are shown at the top of the figure, and below are the molecular
orbitals in an energy-level diagram.
Resonance and Aromaticity
The familiar properties of benzene illustrate the characteristics of aromatic com-
pounds. Benzene is much less reactive toward electrophiles, such as molecular
halogens, than are simple olefins; and the heat evolved on hydrogenation is less
by 37 kcal mole-l than predicted for a cyclic C6H6 with three localized ethylene-
type double bonds. Furthermore, the nuclear magnetic resonance spectrum of
benzene and its derivatives shows the protons bonded to the ring to be experienc-
ing a stronger effective magnetic field than do protons attached to simple
olefins.
As we have seen, these properties are accounted for in the resonance picture
by modifying the model through inclusion of a second structure with double
bonds in the alternative locations.