Page 45 - Mechanism and Theory in Organic Chemistry
P. 45
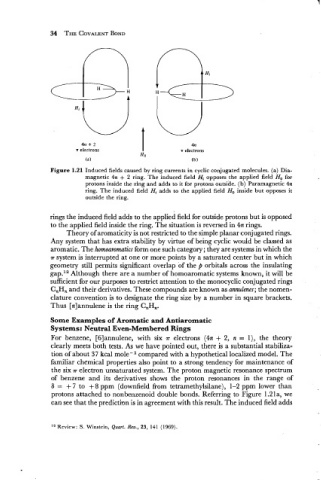
4n + 2 4n
n electrons n electrons
Figure 1.21 Induced fields caused by ring currents in cyclic conjugated molecules. (a) Dia-
magnetic 4n + 2 ring. The induced field Hi opposes the applied field Ho for
protons inside the ring and adds to it for protons outside. (b) Paramagnetic 4n
ring. The induced field Hi adds to the applied field Ho inside but opposes it
outside the ring.
rings the induced field adds to the applied field for outside protons but is opposed
to the applied field inside the ring. The situation is reversed in 4n rings.
Theory of aromaticity is not restricted to the simple planar conjugated rings.
Any system that has extra stability by virtue of being cyclic would be classed as
aromatic. The homoaromatics form one such category; they are systems in which the
.rr system is interrupted at one or more points by a saturated center but in which
geometry still permits significant overlap of the p orbitals across the insulating
gap.12 Although there are a number of homoaromatic systems known, it will be
sufficient for our purposes to restrict attention to the monocyclic conjugated rings
CnHn and their derivatives. These compounds are known as annulenes; the nomen-
clature convention is to designate the ring size by a number in square brackets.
Thus [nlannulene is the ring CnH,.
Some Examples of Aromatic and Antiaromatic
Systems: Neutral Even-Membered Rings
For benzene, [6]annulene, with six .rr electrons (4n + 2, n = l), the theory
clearly meets both tests. As we have pointed out, there is a substantial stabiliza-
tion of about 37 kcal mole-I compared with a hypothetical localized model. The
familiar chemical properties also point to a strong tendency for maintenance of
the six .rr electron unsaturated system. The proton magnetic resonance spectrum
of benzene and its derivatives shows the proton resonances in the range of
6 = + 7 to + 8 ppm (downfield from tetramethylsilane), 1-2 ppm lower than
protons attached to nonbenzenoid double bonds. Referring to Figure 1.21a, we
can see that the prediction is in agreement with this result. The induced field adds
l2 Review: S. Winstein, Quart. Rev., 23, 141 (1969).