Page 49 - Mechanism and Theory in Organic Chemistry
P. 49
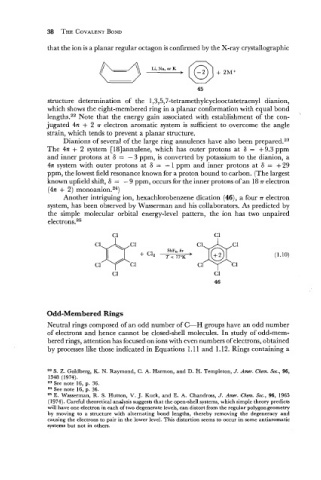
that the ion is a planar regular octagon is confirmed by the X-ray crystallographic
@)
Li, Na, or K
c-9 - +.-+
structure determination of the 1,3,5,7-tetramethylcyclooctatetraenyl dianion,
which shows the eight-membered ring in a planar conformation with equal bond
'
lengths.22 Note that the energy gain associated with establishment of the con-
jugated 4n + 2 rr electron aromatic system is sufficient to overcome the angle
strain, which tends to prevent a planar structure.
Dianions of several of the large ring annulenes have also been prepared.23
The 4n + 2 system [18]annulene, which has outer protons at 8 = +9.3 ppm
and inner protons at 8 = - 3 ppm, is converted by potassium to the dianion, a
4n system with outer protons at 8 = - 1 ppm and inner protons at 8 = + 29
ppm, the lowest field resonance known for a proton bound to carbon. (The largest
known upfield shift, 8 = - 9 ppm, occurs for the inner protons of an 18 rr electron
(4n + 2) monoani~n.~~)
Another intriguing ion, hexachlorobenzene dication (46), a four rr electron
system, has been observed by Wasserman and his collaborators. As predicted by
the simple molecular orbital energy-level pattern, the ion has two unpaired
electrons.25
Odd-Membered Rings
Neutral rings composed of an odd number of C-H groups have an odd number
of electrons and hence cannot be closed-shell molecules. In study of odd-mem-
bered rings, attention has focused on ions with even numbers of electrons, obtained
by processes like those indicated in Equations 1.11 and 1.12. Rings containing a
aa S. Z. Goldberg, K. N. Raymond, C. A. Harmon, and D. PI. Templeton, J. Amer. Chem. Sac., 96,
1348 (1974).
23 See note 16, p. 36.
24 See note 16, p. 36.
25 E. Wasserman, R. S. Hutton, V. J. Kuck, and E. A. Chandross, J. Amer. Chem. Sac., 96, 1965
(1974). Careful theoretical analysis suggests that the open-shell systems, which simple theory predicts
will have one electron in each of two degenerate levels, can distort from the regular polygongeometry
by moving to a structure with alternating bond lengths, thereby removing the degeneracy and
causing the electrons to pair in the lower level. This distortion seems to occur in some antiaromatic
systems but not in others.