Page 48 - Mechanism and Theory in Organic Chemistry
P. 48
Aromaticity 37
(These latter substances are actually intermediates in the preparation of the
annulenes themselves.)
These large rings, even the 4n + 2 ones, do not show the kind of chemical
stability that benzene has, although [18]annulene does undergo electrophilic
substitutions. Ring currents provide the most useful criterion for testing their
aromaticity. The molecules have protons both inside and outside the ring. Con-
formational equilibria such as those indicated in 39 and 40 exchange the inner
and outer protons rapidly at room temperature, but at lower temperatures the
rates are sufficiently slow that the two types of proton can be observed. The
spectra provide a dramatic confirmation of theory. The [14], [18], and [22]annu-
lenes, 4n + 2 systems, have outside proton resonances between about 6 = + 7.8
and 6 = +9.6 ppm, shifts somewhat larger than those in benzene, whereas the
inside protons appear between 6 = - 0.4 and 6 = - 3 ppm.17 (Positive 6 values
are downfield from tetramethylsilane (TMS) ; negative 6 values are upfield.)
The 4n rings [16] and [24]annulene have outside protons at 6 = +4.7 to
6 = + 5.3 ppm and inside protons at much lower field, 6 = + 10 to 8 = + 12
PPm.
Even-Membered Rings:
Cations and Anions
Addition of two electrons to, or removal of two electrons from, a 4n antiaromatic
ring converts it to a 4n + 2 system, which should be aromatic. Several examples
of such ions are known.
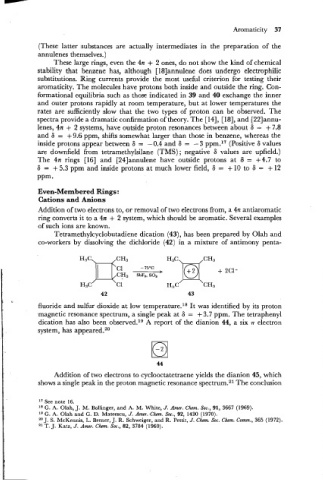
Tetramethylcyclobutadiene dication (43), has been prepared by Olah and
co-workers by dissolving the dichloride (42) in a mixture of antimony penta-
fluoride and sulfur dioxide at low temperature.l8 It was identified by its proton
magnetic resonance spectrum, a single peak at 6 = + 3.7 ppm. The tetraphenyl
dication has also been observed.lg A report of the dianion 44, a six .rr electron
system, has appeared.20
Addition of two electrons to cyclooctatetraene yields the dianion 45, which
shows a single peak in the proton magnetic resonance spectrum.21 The conclusion
l7 See note 16.
l8 G. A. Olah, J. M. Bollinger, and A. M. White, J. Amer. Chem. Sac., 91, 3667 (1969).
l9 G. A. Olah and G. D. Mateescu, J. Amer. Chem. Sac., 92, 1430 (1970).
ao J. S. McKennis, L. Brener, J. R. Schweiger, and R. Pettit, J. Chem. Sac. Chem. Comm., 365 (1972).
21 T. J. Katz, J. Amer. Chem. Sac., 82, 3784 (1960).