Page 46 - Mechanism and Theory in Organic Chemistry
P. 46
Aromaticity 35
1. . '.
outside the ring so that a smaller applied field is required to reach resonance,
hence a downfield shift. There is, of course, no possibility of having a proton inside
a ring this small.
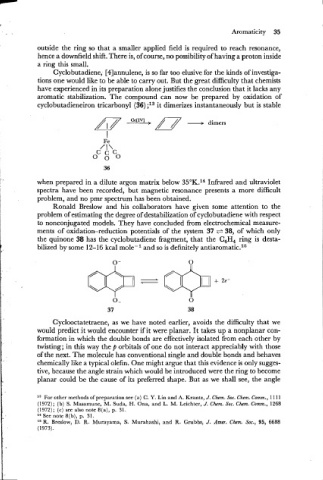
Cyclobutadiene, [4]annulene, is so far too elusive for the kinds of investiga-
tions one would like to be able to carry out. But the great difficulty that chemists
have experienced in its preparation alone justifies the conclusion that it lacks any
aromatic stabilization. The compound can now be prepared by oxidation of
cyclobutadieneiron tricarbonyl (36) ;I3 it dimerizes instantaneously but is stable
dimers
when prepared in a dilute argon matrix below 35"K.14 Infrared and ultraviolet
spectra have been recorded, but magnetic resonance presents a more difficult
problem, and no pmr spectrum has been obtained.
Ronald Breslow and his collaborators have given some attention to the
problem of estimating the degree of destabilization of cyclobutadiene with respect
to nonconjugated models. They have concluded from electrochemical measure-
ments of oxidation-reduction potentials of the system 37 + 38, of which only
the quinone 38 has the cyclobutadiene fragment, that the C H ring is desta-
4
P bilized by some 12-16 kcal mole- l and so is definitely antiaromatic.16
Cyclooctatetraene, as we have noted earlier, avoids the difficulty that we
would predict it would encounter if it were planar. It takes up a nonplanar con-
formation in which the double bonds are effectively isolated from each other by
twisting; in this way the p orbitals of one do not interact appreciably with those
of the next. The molecule has conventional single and double bonds and behaves
chemically like a typical olefin. One might argue that this evidence is only sugges-
tive, bzcause the angle strain which would be introduced were the ring to become
planar could be the cause of its preferred shape. But as we shall see, the angle
L
l3 For other methods of preparation see (a) C. Y. Lin and A. Krantz, J. Chem. Soc. Chem. Comm., 11 11
(1972); (b) S. Masamune, M. Suda, H. Ona, and L. M. Leichter, J. Chem. Soc. Chem. Comm., 1268
(1972) ; (c) see also note 8(a), p. 31.
l4 See note 8(b), p. 31.
R. Breslow, D. R. Murayama, S. Murahashi, and R. Grubbs, J. Amer. Chem. Soc., 95, 6688
(1973).