Page 43 - Mechanism and Theory in Organic Chemistry
P. 43
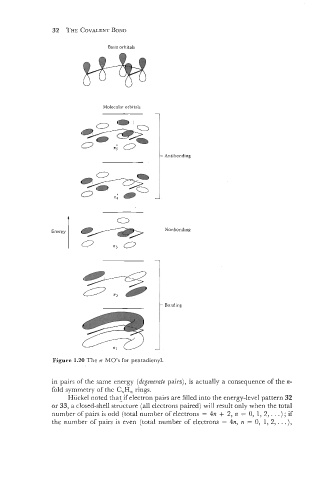
Basis orbitals
Molecular orbitals
- Antibonding
Energy
1 Bonding
Figure 1.20 The n MO's for pentadienyl. Nonbonding
in pairs of the same energy (degenerate pairs), is actually a consequence of the n-
fold symmetry of the CnHn rings.
Hiickel noted that if electron pairs are filled into the energy-level pattern 32
or 33, a closed-shell strGcture (all electrons paired) will result only when the total
number of pairs is odd (total number of electrons = 4n + 2, n = 0, 1, 2, . . .) ; if
the number of pairs is even (total number of electrons = 4n, n = 0, 1, 2,. . .),