Page 39 - Mechanism and Theory in Organic Chemistry
P. 39
The next-higher ener~--MQis
--- -
-
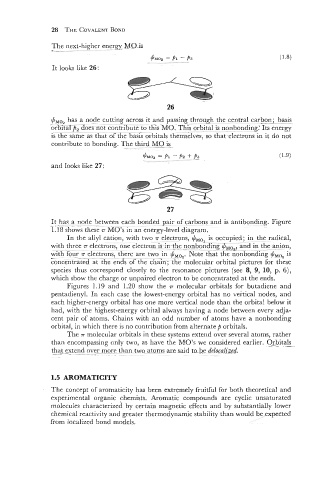
It looks like 26:
has a node cutting across it and passing through the central carbon; basis
a,!~,,,
orbital p, ;ides not contribute to th;s MO. his orbital is nonbonding: Its energy
is the same as that of the basis orbitals themselves: so that electrons in it do not
contribute to bonding. The third-MB is-
-
- _
#MO~ = PI - P2 + @3
-_ - -
and looks like 27:
It has a node between each bonded pair of carbons and is antibonding. Figure
-1.18 shows these n MO's in an energy-level diagram.
In the ally1 cation, with two .rr electrons, a,!JMoI is occupied; in the radical,
in the anion,
with three x electrons, one electron is in the nonbonding $,,,-and
-_
withfour n electrons, there are two in $,,,.
Note that the nonbonding $,,, is
-
-
concentrated at the ends of the chah; the molecular orbital pictures for these
species thus correspond closely to the resonance pictures (see 8, 9, 10, p. 6),
which show the charge or unpaired electron to be concentrated at the ends.
Figures 1.19 and 1.20 show the n molecular orbitals for butadiene and
pentadienyl. In each case the lowest-energy orbital has no veitical nodes, and
each higher-energy orbital has one more vertical node than the orbital below it
had, with the highest-energy orbital always having a node between every adja-
cent pair of atoms. Chains with an odd number of atoms have a nonbonding
orbital, in which there is no contribution from alternate p orbitals.
The n molecular orbitals in these systems extend over several atoms, rather
than encompassing only two, as have the MO's we considered earlier. Orbitals
that ex_te>d ovey_more than two atoms are said to_bedeloc_alized.
1.5 AROMATICITY
The concept of aromaticity has been extremely fruitful for both theoretical and
experimental organic chemists. Aromatic compounds are cyclic unsaturated
molecules characterize8 by certain magnetic effects and by substantially lower
chemical reactivity and greater thermodynamic stability than would be expec~ed
from localized bond models.