Page 37 - Mechanism and Theory in Organic Chemistry
P. 37
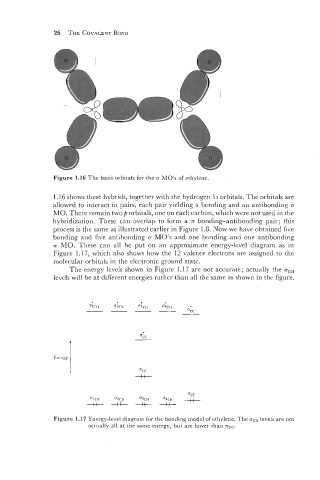
Figure 1.16 The basis orbitals for the a MO's of ethylenc.
1.16 shows these hybrids, together with the hydrogen 1s orbitals. The orbitals are
allowed to interact in pairs, each pair yielding a bonding and an antibonding o
MO. There remain twop orbitals, one on each carbon, which were not used in the
hybridization. These can overlap to form a T bonding-antibonding pair; this
process is the same as illustrated earlier in Figure 1.8. Now.we have obtained five
bonding and five antibonding o MO's and one bonding and one antibonding
T MO. These can all be put on an approximate energy-level diagram as in
Figure 1.17, which also shows how the 12 valence electrons are assigned to the
molecular orbitals in the electronic ground state.
The energy levels shown in Figure 1.17 are not accurate; actually the o,,
levels will be at different energies rather than all the same as shown in the figure.
Figure 1.17 Energy-level diagram for the bonding model of ethylene. The ow levels are not
actually all at the same energy, but are lower than TCC.