Page 32 - Mechanism and Theory in Organic Chemistry
P. 32
Hybrid Orbitals 21
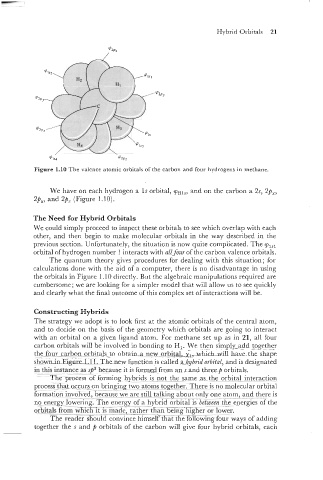
Figure 1.10 The valence atomic orbitals of the carbon and four hydrogens in methane.
and on the carbon a Zs, Zp,,
We have on each hydrogen a 1s orbital, y,,,,
Zp,, and 2p, (Figure 1.10).
The Need for Hybrid Orbitals
We could simply proceed to inspect these orbitals to see which overlap with each
other, and then begin to make molecular orbitals in the way described in the
previous section. Unfortunately, the situation is now quite complicated. The y,,,
orbital of hydrogen number 1 interacts with all four of the carbon valence orbitals.
The quantum theory gives procedures for dealing with this situation; for
calculations done with the aid of a computer, there is no disadvantage in using
the orbitals in Figure 1.10 directly. But the algebraic manipulations required are
cumbersome; we are looking for a simplcr model that will allow us to see quickly
and clearly what the final outcome of this complex set of interactions will be.
Constructing Hybrids
The strategy we adopt is to look first at the atomic orbitals of the central atom,
and to decide on the basis of the geometry which orbitals are going to interact
with an orbital on a given ligand atom. For methane set up as in 21, all four
carbon orbitals will be involved in bonding to H,. We then sirgly-add together
the four carbon _orJbitals-to obtain-a new_-orbital: Gywhich4viLL have the shape
shownin-Fkure-1.1-1. Thenew function is called %hybrid orbital, and is designated
in this instance as sp3 because it is form-ed-from an s and three p orbitals.
up
The process of forming hybrids is not the sameas the o_r_bdal intcraction
process~h_a_t occ-urs on bringing two - atoms together. There is no molecular orbital
-
-
formation involved, because_weeare still talking about only one atom, and there is
no-energy lowering. The energy of a hybridorbital is between the energies of the
orbitals from-trmadk -- J-- rath<ihan~~fi~~Kiher lower.
or
-- -
-
The reader should convince himselfthat3lEGlfowing four ways of adding
together the s and p orbitals of the carbon will give four hybrid orbitals, each