Page 28 - Mechanism and Theory in Organic Chemistry
P. 28
Molecular Orbitals 17
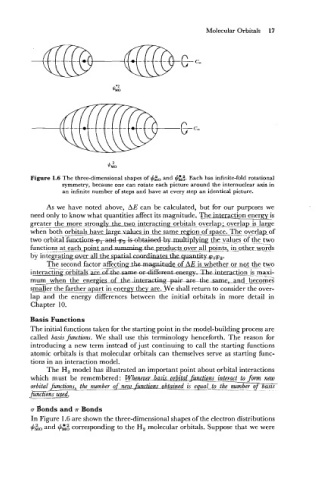
Figure 1.6 The three-dimensional shapes of #so and #::. Each has infinite-fold rotational
symmetry, because one can rotate each picture around the internuclear axis in
an infinite number of steps and have at every step an identical picture.
As we have noted above, AE can be calculated, but for our purposes we
need only to know what quantities affect its magnitude. xhehaction energy is
reater the more stron~ly the two interacting orbitals overlap~c.ye_r_Ia~ is large
g . __ ---
when both orbitals have yahe.s in the same region of space. T&e,v_-ay-of
two orbit~~~~-~w~2~if-ObtaW:d-~-~pY;ng. of thetwo
&e-valEs
functions at each ~oint pr oducts over all poi-nns, in-ot_herrw&s
..
.
by integratingmer all t h ~ ~ y - P , 4 p , .
The --. second factor - affecting- e of AE is whether or not the tky-
.
.
interactinp orbitaTsare nf t h p c t i o n - i s -maxi-
mum when the energies of the intPr;lrtiffP.- and_ becor@$
--.-
smaller the farthchapart.i.nincrgy they are. We shall return to consider the over-
lap and the energy differences between the initial orbitals in more detail in
Chapter 10.
Basis Functions
The initial functions taken for the starting point in the modebbuilding process are
called basis functions. We shall use this terminology henceforth. The reason for
introducing a new term instead of just continuing to call the starting functions
atomic orbitals is that molecular orbitals can themselves serve as starting func-
tions in an interaction model.
The H, model has illustrated an important point about orbital interactions
which must be remembered : -Whenev~rhis orbital d&&ionr interact to form new
orbital junctions, the number new f i r o btained is equal-to the number bmzs
----
mans used.
aBondsand~Bonds
In Figure 1.6 are shown the three-dimensiona1 shapes of the electron distributions
and
#iO $5; corresponding to the H, molecular orbitals. Suppose that we were