Page 26 - Mechanism and Theory in Organic Chemistry
P. 26
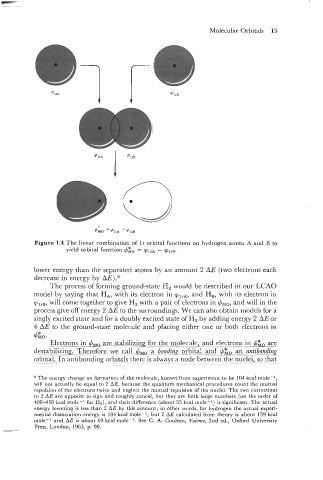
Molecular Orbitals 15
Figure 1.4 The linear combination of Is orbital functions on hydrogen atoms A and B to
yield orbital function $Go =
- tpl,~.
lower energy than the separated atoms by an amount 2 AE (two electrons each
decrease in energy by AE).6
The process of forming ground-state Hz would be described in our LCAO
and H,, with its electron in
model by saying that HA, with its electron in 91,,,,
and will in the
will come together to give Hz with a pair of electrons in
$,,,
91,,,,
process give off energy 2 AE to the surroundings. We can also obta~n models for a
singly excited state and for a doubly excited state of H, by adding energy 2 AE or
4 AE to the ground-state molecule and placing either one or both electrons in
*Go.
Elec-abilking
for the molecule, and ---- electrons in 4;" are
--
destabilizing. Ther-efore we-~all_+~ bonding- ohi~a! and $$a t;,n-g&bonding-
-a
orbital. In antibonding orbitqls there is - always a node between the nuclei, so that
-
--
-
- -
The energy change on formation of the molecule, known from experiment to be 104 kcal mole-',
will not actually be equal to 2 AE, because the quantum mechanical procedures count the mutual
repulsion of the electrons twice and neglect the mutual repulsion of the nuclei. The two corrections
to 2 AE are opposite in sign and roughly cancel, but they are both large numbers (on the order of
400450 kcal mole-' for Hz), and their difference (about 35 kcal mole-') is significant. The actual
energy lowering is less than 2 AE by this amount; in other words, for hydrogen the actual experi-
mental dissociation energy is 104 kcal mole-', but 2 AE calculated from theory is about 139 kcal
mole-' and AE is about 69 kcal mole-'. See C. A. Coulson, Valence, 2nd ed., Oxford University
Press, London, 1963, p. 90.