Page 33 - Mechanism and Theory in Organic Chemistry
P. 33
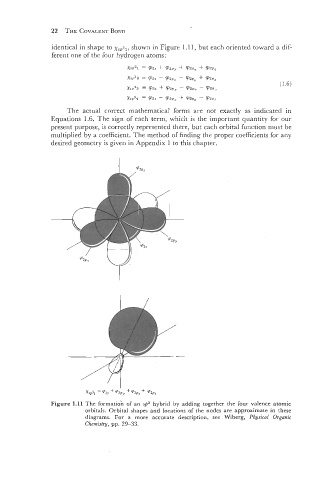
identical in shape to ,ySP31, shown in Figure 1.11, but each oriented toward a dif-
ferent one of the [our hydrogen atoms:
The actual correct mathematical forms are not exactly as indicated in
Equations 1.6. The sign of each term, which is the important quantity for our
present purpose, is correctly represented there, but each orbital function must be
multiplied by a coefficient. The method of finding the proper coefficients for any
desired geometry is given in Appendix 1 to this chapter.
Figure 1.11 The formation of an sp3 hybrid by adding together the four valence atomic
orbitals. Orbital shapes and locations of the nodes are approximate in these
diagrams. For a more accurate description, see Wiberg, Physical Organic
Chemistry, pp. 29-33.