Page 50 - Mechanism and Theory in Organic Chemistry
P. 50
Aromaticity 39
hetero atom that contributes two electrons to the n system are isoelectronic with
the C-H ring anion of the same size. Rings containing one carbonyl group
resemble the C-H ring cation of the same size because the electron-withdrawing
carbonyl oxygen leaves the carbonyl carbon electron-deficient. According to
-
theory, rings -- of - -a 3, 7, 11, . . . mem-bers should yield aromatic cations and anti-
--
aromatic anions, whereas rings of 5, 9, 13, . . . members should give aromatic
-.
anions and antiaromatic'cations.
.
-
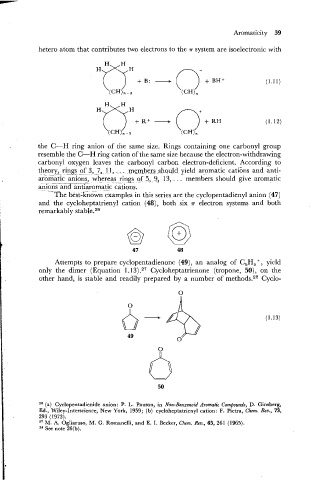
The best-known examples in this series are the cyclopentadienyl anion (47)
and the cycloheptatrienyl cation (48), both six n electron systems and both
remarkably stable.26
Attempts to prepare cyclopentadienone (49), an analog of C5H5 + , yield
only the dimer (Equation 1.13) .27 Cycloheptatrienone (tropone, 50): on the
other hand, is stable and readily prepared by a number of methods.28 Cyclo-
(a) Cyclopentadienide anion: P. L. Pauson, in Non-Benzenoid Aromatic Compounds, D. Ginsberg,
Ed., Wiley-Interscience, New York, 1959; (b) cycloheptatrienyl cation: F. Pietra, Chem. Rev., 73,
293 (1973).
27 M. A. Ogliaruso, M. G. Romanelli, and E. I. Becker, Chem. Rev., 65, 261 (1965).
as See note 26(b).