Page 51 - Mechanism and Theory in Organic Chemistry
P. 51
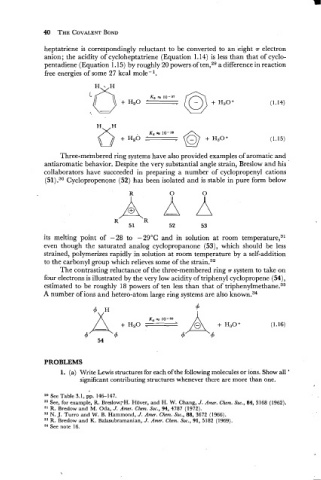
heptatriene is correspondingly reluctant to be converted to an eight .rr electron
anion; the acidity of cycloheptatriene (Equation 1.14) is less than that of cyclo-
pentadiene (Equation 1.15) by roughly 20 powers of ten,29 a difference in reaction
free energies of some 27 kcal mole-l.
Three-meinbered ring systems have also provided examples of aromatic and
antiaromatic behavior. Despite the very substantial angle strain, Breslow and his '
collaborators have succeeded in re paring a number of cyclopropenyl cations
(51).30 Cyclopropenone (52) has been isolated and is stable in pure form below
its melting point of -28 to -2g°C and in solution at room temperat~re,~~
even though the saturated analog cyclopropanone (53), which should be less
strained, polymerizes rapidly in solution at room temperature by a self-addition
to the carbonyl group which relieves some of the strain.32
The contrasting reluctance of the three-membered ring T system to take on
four electrons is illustrated by the very low acidity of triphenyl cyclopropene (54),
estimated to be roughly 18 powers of ten less than that of tri~henylmethane.~~
A number of ions and hetero-atom large ring systems are also known.34
PROBLEMS
1. (a) Write Lewis structures for each of the following molecules or ions. Show all '
. .
significant contributing structures whenever there are more than one.
20 See Table 3.1, pp. 146-147.
30 See, for example, R. Bres1ow;H. Hover, and H. W. Chang, J. Amer. Chem. Soc., 84, 3168 (1962).
31 R. Breslow and M. Oda, J. Amer. Chem. Soc., 94, 4787 (1972).
3a N. J. Turro and W. B. Hammond, J. Amer. Chem. Soc., 88, 3672 (1966).
33 R. Breslow and K. Balasubramanian, J. Amer. Chem. Soc., 91, 5182 (1969).
34 See note 16.