Page 56 - Mechanism and Theory in Organic Chemistry
P. 56
45
Hybrid Orbitals
properties of a hybrid are entirely determined by the relative contributions of
Px, Pv, and p,. It is therefore convenient to think of three-dimensional vectors
oriented along the directions in which we wish our hybrids to point. (It is
important to understand that we are now talking about vectors in ordinary
three-dimensional space.)
These vectors can be written in terms of vectors along the x, y, and z direc-
tions using polar coordinates 8 and c$, as indicated in Equation A1.6 and illus-
trated in Figure Al. 1.
(A1 -6)
y
x
v = sin @ cos 4 + sin @sin 4 + cos @ z
The reader can verify that v defined in this way is normalized (of unit length).
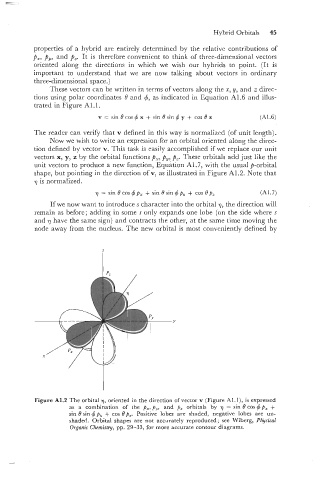
Now we wish to write an expression for an orbital oriented along the direc-
tion defined by vector v. This task is easily accomplished if we replace our unit
vectors x, y, z by the orbital functions p,, p,? P,. These orbitals add just like the
unit vectors to produce a new function, Equation A1.7, with the usual p-orbital
shape, but pointing in the direction of v, as illustrated in Figure A1.2. Note that
7 is normalized.
(A1.7)
7 = sin 0 cos +p, + sin 0 sin p, + cos Bp,
If we now want to introduce s character into the orbital q, the direction will
remain as before; adding in some s only expands one lobe (on the side where s
and 7 have the same sign) and contracts the other, at the same time moving the
node away from the nucleus. The new orbital is most conveniently defined by
Figure A1.2 The orbital 7, oriented in the direction of vector v (Figure Al.l), is expressed
as a combination of the p,,p,, and p, orbitals by 7 = sin 0 cos+p, +
sin B sin 4 p, + cos Bp,. Positive lobes are shaded, negative lobes are un-
shaded. Orbital shapes are not accurately reproduced; see Wiberg, Physical
Organic Chemistry, pp. 29-33, for more accurate contour diagrams.