Page 57 - Mechanism and Theory in Organic Chemistry
P. 57
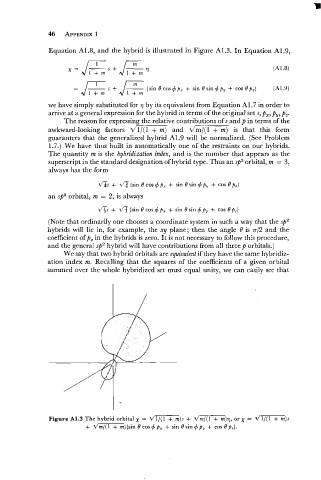
Equation A1.8, and the hybrid is illustrated in Figure A1.3. In Equation A1.9,
- s + Jx cos 4 + sin 6' sin 4 py + cos 6' pi} (A1.9)
-
p,
6'
{sin
I+m l+m
we have simply substituted for q by its equivalent from Equation A1.7 in order to
arrive at a ginkral expression for the hybEid in terms of the original set s, p,, P,, p,.
The reason for expressing the relative contributions of s and P in terms of the
awkward-looking factors d1/(1 + m) and dm/( 1 + m) is that this form
-
guarantees that the generalized hybrid A1.9 will be normalized. (See Problem
1.7.) We have thus Luilt in automaticallv one of the restraints on'our hybrids.
The quantity m is the hybridization index, and is the number that appears as the
superscript in the standard designation of hybrid type. Thus an sp3 orbital, m = 3,
always has the form
4s + {sin 6' cos 4 p, + sin 6' sin 4 + cos 6' p,}
p,
an sp2 orbital, m = 2, is always
p,
4s + x/3 {sin 6' cos 4 + sin 6' sin 4 p, + cos 6' p,}
(Note that ordinarily one chooses a coordinate system in such a way that the sp2
hybrids will lie in, for example, the xy plane; then the angle 6 is 7r/2 and the
coefficient ofp, in the hybrids is zero. It is not necessary to follow this procedure,
and the general sp2 hybrid will have contributions from all three p orbitals.)
We say that two hybrid orbitals are equivalent if they have the same hybridiz-
ation index m. Recalling that the squares of the coefficients of a given orbital
summed over the whole hybridized set must equal unity, we can easily see that
Figure A1.3 The hybrid orbital x = d1/(1 + m)s + dm/(l + m)', or x = d1/(1 + m)s
+ dm/(l + m){sin 6' cos 4 p, + sin 6' sin 4 p, + cos 6' p,}.