Page 59 - Mechanism and Theory in Organic Chemistry
P. 59
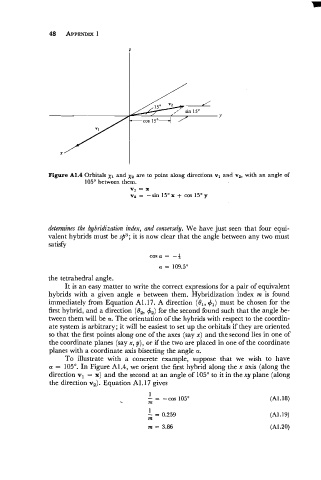
Figure A1.4 Orbitals x1 and x2 are to point along directions vl and v2, with an angle of
105' between them.
v1 = X
v2 = -sin 15Ox + cos 15Oy
determines the hybridization index, and conversely. We have just seen that four equi-
valent hybrids must be sp3; it is now clear that the angle between any two must
satisfy
the tetrahedral angle.
It is an easy matter to write the correct expressions for a pair of equivalent
hybrids with a given angle a between them. Hybridization index m is found
immediately from Equation A1.17. A direction (el, 4,) must be chosen for the
first hybrid, and a direction (O,, 4,) for the second found such that the angle be-
tween them will be a. The orientation of the hybrids with respect to the coordin-
ate system is arbitrary; it will be easiest to set up the orbitals if they are oriented
so that the first points along one of the axes (say x) and the second lies in one of
the coordinate planes (say x, y), or if the two are placed in one of the coordinate
planes with a coordinate axis bisecting the angle a.
To illustrate with a concrete example, suppose that we wish to have
a = 105'. In Figure A1.4, we orient the first hybrid along the x axis (along the
direction v1 = x) and the second at an angle of 105" to it in the xy plane (along
the direction v,). Equation A1.17 gives
1
- - = - cos 105'
rn
1
- = 0.259
m
m = 3.86