Page 253 - Mechanism and Theory in Organic Chemistry
P. 253
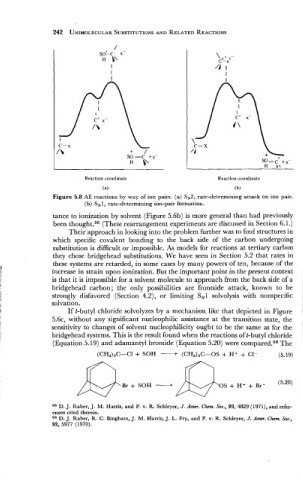
I
I
I I
I
C-x C-X
/\ + /
SO-C +x
H I\ so-c b\ +x-
H
Reaction coordinate Reaction coordinate
(a) (b)
Figure 5.8 All reactions by way of ion pairs. (a) SN2, rate-determining attack on ion pair.
(b) SN 1, rate-determining ion-pair formation.
tance to ionization by solvent (Figure 5.6b) is more general than had previously
been (These rearrangement experiments are discussed in Section 6.1.)
Their approach in looking into the problem further was to find structures in
which specific covalent bonding to the back side of the carbon undergoing
substitution is difficult or impossible. As models for reactions at tertiary carbon
they chose bridgehead substitutions. We have seen in Section 5.2 that rates in
these systems are retarded, in some cases by many powers of ten, because of the
increase in strain upon ionization. But the important point in the present context
is that it is impossible for a solvent molecule to approach from the back side of a
bridgehead carbon; the only possibilities are frontside attack, known to be
strongly disfavored (Section 4.2), or limiting S,1 solvolysis with nonspecific
solvation.
If t-butyl chloride solvolyzes by a mechanism like that depicted in Figure
5.6c, without any significant nucleophilic assistance at the transition state, the
sensitivity to changes of solvent nucleophilicity ought to be the same as for the
bridgehead systems. This is the result found when the reactions of t-butyl chloride
(Equation 5.19) and adamantyl bromide (Equation 5.20) were c~mpared.~The
(CH3)3C-CI + SOH --+ (CH3)3C-OS + H+ + C1- (5.19)
as D. J. Raber, J. M. Harris, and P. v. R. Schleyer, J. Am. Chem. Sot., 93, 4829 (1971), and refer-
ences cited therein.
D. J. Raber, R. C. Bingham, J. M. Harris, J. L. Fry, and P. v. R. Schleyer, J. Am. Chem. Sot.,
92, 5977 (1970).