Page 248 - Mechanism and Theory in Organic Chemistry
P. 248
Mechanisms Intermediate Between SN1 and SN2 237
on the basis of detailed molecular orbital calculations to be linear if R, and R,
are the same,85 but will probably not be exactly linear if R, # R,.86
-
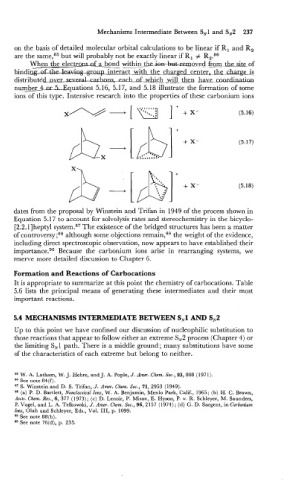
When the electransaf_ a bond within th&xA&xemved from the &of
bindi-pi&ettwiththg. c-harged center. the -is
h
~
d i ~ t r i b u t Z d d c -wd then haye coordination
num~Uquations 5.16, 5.17, and 5.18 illustrate the formation of some
ions of this type. Intensive research into the properties of these carbonium ions
dates from the proposal by Winstein and Trifan in 1949 of the process shown in
Equation 5.17 to account for solvolysis rates and stereochemistry in the bicyclo-
[2.2. llheptyl system.87 The existence of the bridged structures has been a matter
of contr~versy;~~ although some objections remain,89 the weight of the evidence,
including direct spectroscopic observation, now appears to have established their
importan~e.~~ Because the carbonium ions arise in rearranging systems, we
reserve more detailed discussion to Chapter 6.
Formation and Reactions of Carbocations
It is appropriate to summarize at this point the chemistry of carbocations. Table
5.6 lists the principal means of generating these intermediates and their most
important reactions.
5.4 MECHANISMS INTERMEDIATE BETWEEN S,1 AND S,2
Up to this point we have confined our discussion of nucleophilic substitution to
those reactions that appear to follow either an extreme S,2 process (Chapter 4) or
the limiting S,1 path. There is a middle ground; many substitutions have some
of the characteristics of each extreme but belong to neither.
O5 W. A. Latham, W. J. Hehre, and J. A. Pople, J. Amer. Chem. Soc., 93, 808 (1971).
See note 84(f).
O7 S. Winstein and D. S. Trifan, J. Amer. Chem. Soc., 71, 2953 (1949).
(a) P. D. Bartlett, Nonclassical Ions, W. A. Benjamin, Menlo Park, Calif., 1965; (b) H. C. Brown,
Accts. Chem. Res., 6, 377 (1973); (c) D. Lenoir, P. Mison, E. Hyson, P. v. R. Schleyer, M. Saunders,
P. Vogel, and L. A. Telkowski, J. Amer. Chem. Soc., 96, 2 157 (1974); (d) G. D. Sargent, in Carbonium
Ions, Olah and Schleyer, Eds., Vol. 111, p. 1099.
See note 88(b).
O0 See note 76(d), p. 235.