Page 243 - Mechanism and Theory in Organic Chemistry
P. 243
logk -5
0 1 2 3
log k~ lkw
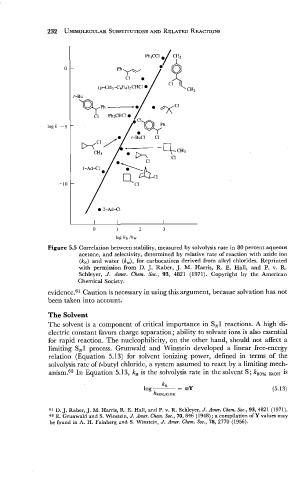
Figure 5.5 Correlation between stability, measured by solvolysis rate in 80 percent aqueous
acetone, and selectivity, determined by relative rate of reaction with azide ion
(k,) and water (k,), for carbocations derived from alkyl chlorides. Reprinted
with permission from D. J. Raber, J. M. Harris, R. E. Hall, and P. v. R.
Schleyer, J. Amer. Chem. Soc., 93, 4821 (1971). Copyright by the American
Chemical Society.
evidence.61 Caution is necessary in using this argument, because solvation has not
been taken into account.
The Solvent
The solvent is a component of critical importance in SN1 reactions. A high di-
electric constant favors charge separation; ability to solvate ions is also essential
for rapid reaction. The nucleophilicity, on the other hand, should not affect a
limiting SN1 process. Grunwald and Winstein developed a linear free-energy
relation (Equation 5.13) for solvent ionizing power, defined in terms of the
ani~m.~~ Equation 5.13, k, is the solvolysis rate in the solvent S; k,,% .,,,
solvolysis rate of t-butyl chloride, a system assumed to react by a limiting mech-
is
In
ks
my
=
log -
~BOXE~OH
D. J. Raber, J. M. Harris, R. E. Hall, and P. v. R. Schleyer, J. Amer. Chem. Soc., 93, 4821 (1971).
6a E. Grunwald and S. Winstein, J. Amer. Chem. Soc., 70,846 (1948); a compilation of Y values may
be found in A. H. Fainberg and S. Winstein, J. Amer. Chem. Soc., 78, 2770 (1956).