Page 239 - Mechanism and Theory in Organic Chemistry
P. 239
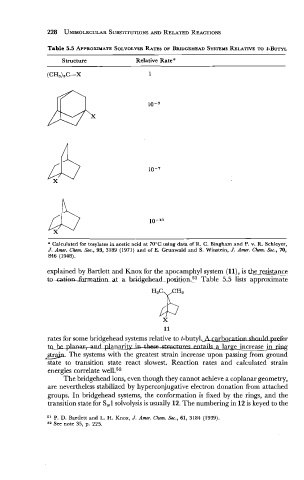
Table 5.5 APPROXIMATE SOLVOLYSIS RATES OF BRIDGEHEAD SYSTEMS RELATIVE t-BUTYL
TO
Structure Relative Rate a
(CH3)3C--X 1
a Calculated for tosylates in acetic acid at 70°C using data of R. C. Bingham and P. v. R. Schleyer,
J. Amr. Chcm. Soc., 93, 3 189 (1 97 1) and of E. Grunwald and S. Winstein, J. Amr. Chcm. Soc., 70,
846 (1948).
explained by Bartlett and Knox for the apocamphyl system (ll), is the resist_ance
to -ca&m.lkrm&at a-bridgehead position.51 Table 5.5 lists approximate
x
11
rates for some bridgehead systems relative to t-buty1.A carbocatlanshnuldprefer
a larlaree increase in ring
&. The systems with the greatest strain increase upon passing from ground
state to transition state react slowest. Reaction rates and calculated strain
energies correlate well.52
The bridgehead ions, even though they cannot achieve a coplanar geometry,
are nevertheless stabilized by hyperconjugative electron donation from attached
groups. In bridgehead systems, the conformation is fixed by the rings, and the
transition state for S,1 solvolysis is usually 12. The numbering in 12 is keyed to the
P. D. Bartlett and L. H. Knox, J. Amer. Chem. Soc., 61, 3184 (1939).
62 See note 35, p. 225.