Page 235 - Mechanism and Theory in Organic Chemistry
P. 235
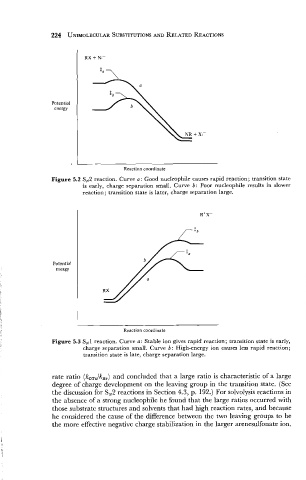
Potential
energy
I
Reaction coordinate
Figure 5.2 SN2 reaction. Curve a: Good nucleophile causes rapid reaction; transition state
is early, charge separation small. Curve b: Poor nucleophile results in slower
reaction; transition state is later, charge separation large.
Potential
energy
- -
Reaction coordinate
Figure 5.3 SN1 reaction. Curve a: Stable ion gives rapid reaction; transition state is early,
charge separation small. Curve b: High-energy ion causes less rapid reaction;
transition state is late, charge separation large.
rate ratio (k,,,,/k,,) and concluded that a large ratio is characteristic of a large
degree of charge development on the leaving group in the transition state. (See
the discussion for S,2 reactions in Section 4.3, p. 192.) For solvolysis reactions in
the absence of a strong nucleophile he found that the large ratios occurred with
those substrate structures and solvents that had high reaction rates, and because
he considered the cause of the difference between the two leaving groups to be
the more effective negative charge stabilization in the larger arenesulfonate ion,