Page 237 - Mechanism and Theory in Organic Chemistry
P. 237
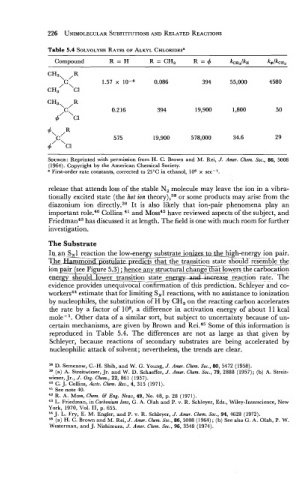
Table 5.4 SOLVOLYSIS RATES OF ALKYL CHLORIDES'
Compound R=H R = CH, R=$ k~~3/k~ k@/kca3
SOURCE: Reprinted with permission from H. C. Brown and M. Rei, J. Amer. Chem. SOC., 86, 5008
(1964). Copyright by the American Chemical Society.
a First-order rate constants, corrected to 25OC in ethanol, 10% x sec-'.
release that attends loss of the stable N, molecule may leave the ion in a vibra-
tionally excited state (the hot ion theory),38 or some products may arise from the
diazonium ion directly.39 It is also likely that ion-pair phenomena play an
important role.40 Collins 41 and Moss42 have reviewed aspects of the subject, and
Friedman4, has discussed it at length. The field is one with much room for further
investigation.
The Substrate
--_ _
I~an SN1 reaction the low-energy substrate ionizes to the high-energy ion pair.
The Hammoni-@stuIZee&cts that the transition state should- resemble the
ion
. pair - (see Figure 5.31; hegce_any structural change tEt lowers -. the carbocation
-
e~ergy should1ower transition state en .- reaction rate.-The
evidence provides unequivocal confirmat=on. ~chle~er and co-
worker~~~ estimate that for limiting SN1 reactions, with no assistance to ionization
by nucleophiles, the substitution of H by CH, on the reacting carbon accelerates
the rate by a factor of lo8, a difference in activation energy of about 11 kcal
mole-'. Other data of a similar sort, but subject to uncertainty because of un-
certain mechanisms, are given by Brown and Rei.45 Some of this information is
reproduced in Table 5.4. The differences are not as large as that given by
Schleyer, because reactions of secondary substrates are being accelerated by
nucleophilic attack of solvent; nevertheless, the trends are clear.
D. Semenow, C.-H. Shih, and W. G. Young, J. Amer. Chem. SOC., 80, 5472 (1958).
39 (a) A. Streitwieser, Jr. and W. D. Schaeffer, J. Amer. Chem. Soc., 79, 2888 (1957); (b) A. Streit-
wieser, Jr., J. Org. Chem., 22, 861 (1957).
40 C. J. Collins, Accts. Chem. Res., 4, 315 (1971).
41 See note 40.
42 R. A. MOSS, Chem. &' Eng. News, 49, No. 48, p. 28 (1971).
43 L. Friedman, in Carbonium Ions, G. A. Olah and P. v. R. Schleyer, Eds., Wiley-Interscience, New
York, 1970, Vol. 11, p. 655.
44 J. L. Fry, E. M. Engler, and P. v. R. Schleyer, J. Amer. Chem. SOC., 94, 4628 (1972).
45 (a) H. C. Brown and M. Rei, J. Amer. Chem. Soc., 86,5008 (1964) ; (b) See also G. A. Olah, P. W.
Westerman, and J. Nishimura, J. Amer. Chem. SOC., 96, 3548 (1974).