Page 240 - Mechanism and Theory in Organic Chemistry
P. 240
Effects of Structure and Solvent 229
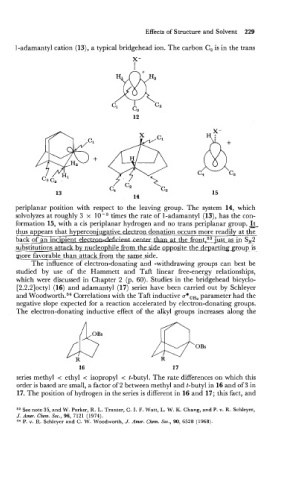
1-adamantyl cation (13), a typical bridgehead ion. The carbon C, is in the trans
periplanar position with respect to the leaving group. The system 14, which
solvolyzes at roughly 3 x times the rate of 1-adamantyl (13), has the con-
formation 15, with a cis periplanar hydrogen and no trans periplanar group. .&
- -
thus appears that hyperconiugative electron donation occurs more readily at tc-
in
back of an inci~ient el-rPnter the fra~t,~3ju_stas SN2
sibstitutions atfack by nucleophile from-thz-side ~~p-osite the_-departins-group is
more favorable than attack from thg s~me-side.
The influence of electron-donating and -withdrawing groups can best be
studied by use of the Hammett and Taft linear free-energy relationships,
which were discussed in Chapter 2 (p. 60). Studies in the bridgehead bicyclo-
[2.2.2]octyl (16) and adamantyl (17) series have been carried out by Schleyer
and Wo~dworth.~~ Correlations with the Taft inductive u*,,~ parameter had the
negative slope expected for a reaction accelerated by electron-donating groups.
The electron-donating inductive efiect of the alkyl groups increases along the
series methyl < ethyl < isopropyl < t-butyl. The rate difierences on which this
order is based are small, a factor of 2 between methyl and t-butyl in 16 and of 3 in
17. The position of hydrogen in the series is difierent in 16 and 17; this fact, and
63 See note 35, and W. Parker, R. L. Tranter, C. I. F. Watt, L. W. K. Chang, and P. v. R. Schleyer,
J. Amer. Chem. Soc., 96, 712 1 (1974).
54 P. V. R. Schleyer and C. W. Woodworth, J. Amer. Chem. Soc., 90, 6528 (1968).