Page 242 - Mechanism and Theory in Organic Chemistry
P. 242
Effects of Structure and Sblvent 231
Reaction coordinate
(a)
Reaction coordinate
(b)
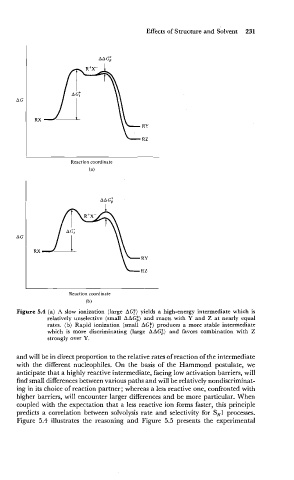
Figure 5.4 (a) A slow ionization (large AGj) yields a high-energy intermediate which is
relatively unselective (small AAG;) and reacts with Y and Z at nearly equal
rates. (b) Rapid ionization (small AGf) produces a more stable intermediate
which is more discriminating (large AAG;) and favors combination with Z
strongly over Y.
and will be in direct proportion to the relative rates of reaction of the intermediate
with the different nucleophiles. On the basis of the Hammond postulate, we
anticipate that a highly reactive intermediate, facing low activation barriers, will
find small differences between various paths and will be relatively nondiscriminat-
ing in its choice of reaction partner; whereas a less reactive one, confronted with
higher barriers, will encounter larger differences and be more particular. When
coupled with the expectation that a less reactive ion forms faster, this principle
predicts a correlation between solvolysis rate and selectivity for S,1 processes.
Figure 5.4 illustrates the reasoning and Figure 5.5 presents the experimental