Page 247 - Mechanism and Theory in Organic Chemistry
P. 247
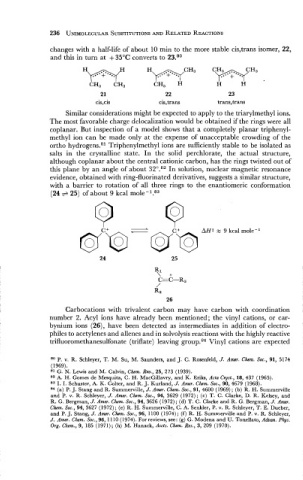
changes with a half-life of about 10 min to the more stable cis,trans isomer, 22,
and this in turn at + 35°C converts to 23.80
.
.
H-ypyH "*C"3 C . . " 3
W
CH, CH, CH3 H H H
Similar considerations might be expected to apply to the triarylmethyl ions.
The most favorable charge delocalization would be obtained if the rings were all
coplanar. But inspection of a model shows that a completely planar triphenyl-
methyl ion can be made only at the expense of unacceptable crowding of the
ortho hydrogens.81 Triphenylmethyl ions are sufficiently stable to be isolated as
salts in the crystalline state. In the solid perchlorate, the actual structure,
although coplanar about the central cationic carbon, has the rings twisted out of
this plane by an angle of about 32°.82 In solution, nuclear magnetic resonance
evidence, obtained with ring-fluorinated derivatives, suggests a similar structure,
with a barrier to rotation of all three rings to the enantiomeric conformation
(24 + 25) of about 9 kcal m01e-l.~~
AH
acD = acB I kcal mole-'
9
Carbocations with trivalent carbon may have carbon with coordination
number 2. Acyl ions have already been mentioned; the vinyl cations, or car-
bynium ions (26), have been detected as intermediates in addition of electro-
philes to acetylenes and allenes and in solvolysis reactions with the highly reactive
trifluoromethanesulfonate (triflate) leaving Vinyl cations are expected
P. v. R. Schleyer, T. M. Su, M. Saunders, and J. C. Rosenfeld, J. Amer. Chem. SOC., 91, 5174
(1969).
G. N. Lewis and M. Calvin, Chem. Rev., 25, 273 (1939).
A. H. Gornes de Mesquita, C. H. MacGillavry, and K. Eriks, Acta Cyst., 18, 437 (1965).
83 I. I. Schuster, A. K. Colter, and R. J. Kurland, J. Amer. Chem. SOC., 90, 4679 (1968).
g4 (a) P. J. Stang and R. Summerville, J. Amer. Chem. SOC., 91, 4600 (1969) ; (b) R. H. Summerville
and P. v. R. Schleyer, J. Amer. Chem. Soc., 94, 3629 (1972); (c) T. C. Clarke, D. R. Kelsey, and
R. G. Bergman, J. Amer. Chem. SOC., 94, 3626 (1972); (d) T. C. Clarke and R. G. Bergman, J. Amer.
Chem. Soc., 94, 3627 (1972); (e) R. H. Surnmerville, C. A. Senkler, P. v. R. Schleyer, T. E. Dueber,
and P. J. Stang, J. Amer. Chem. Soc., 96, 1100 (1974); (f) R. H. Sumrnerville and P. v. R. Schleyer,
J. Amer. Chcm. Soc., 96, 11 10 (1974). For reviews, see: (g) G. Modena and U. Tonellato, Aduan. Phys.
Org. Chem., 9, 185 (1971) ; (h) M. Hanack, Accts. Chem. Res., 3, 209 (1970).