Page 244 - Mechanism and Theory in Organic Chemistry
P. 244
Carbocations 233
the rate in the standard solvent, 80 percent aqueous ethanol; m measures the
sensitivity of the particular system to solvent change; and Y is the ionizing power
of solvent S, determined from t-butyl chloride solvolysis rates by defining m = 1
for this substrate.
In addition to allowing comparison among experiments carried out in dif-
ferent solvents, the m-Y system serves as an important tool for study of mech-
anism. Sensitivity to ionizing power, measured by m, is an index of the degree of
charge separation at the transition state. The ratio of rates in two solvents of
equal Y but different nucleophilicity provides evidence about nucleophilic
assistance by a solvent molecule during the ionization.
Other solvent parameters based on the influence of solvent on electronic
excitation energies have been developed by Ko~ower;~~ Smith, Fainberg, and
and
Win~tein;~~ Dimroth and co-workers.65
Despite its usefulness, the Y parameter system is not without flaw. Like
most linear free-energy correlations, it fails if rigorously applied to compounds of
diverse structural types. Thus when a given substrate is studied in different
solvent systems (for example acetone-water, ethanol-water, acetic acid-formic
acid), slightly different slopes m are 'obtained.66
5.3 CARBOCATIONS
The chemistry of carbocations has been intensively studied, and the literature is
vast. We can do no more here than summarize some of the important features of
the field. A comprehensive review in four volumes covers the area in detail.67
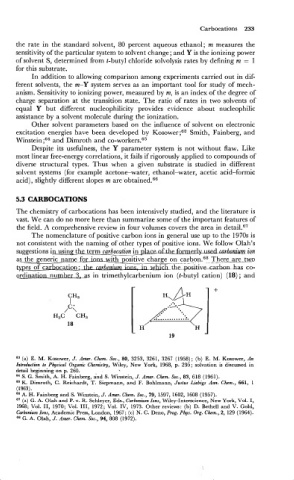
The nomenclature of positive carbon ions in general use up to the 1970s is
not consistent with the naming of other types of positive ions. We follow Olah's
-
suggestions in using the term carbocation in place nf th.efmnzxlymdcazhnUra ion
positive charge on carbon.68 There are two
as the generic name for i-ith . .
types of carbocation: the _carbenzum-&L-@&ivecarbnn has co-
qdinatioanumber 3, as in trimethylcarbenium ion (t-butyl cation) (18) ; and
83 (a) E. M. Kosower, J. Amer. Chem. SOC., 80, 3253, 3261, 3267 (1958); (b) E. M. Kosower, An
Introduction to Physical Organic Chemistry, Wiley, New York, 1968, p. 295; solvation is discussed in
detail beginning on p. 260.
" S. G. Smith, A. H. Fainberg, and S. Winstein, J. Amer. Chem. SOC., 83, 618 (1961).
K. Dimroth, C. Reichardt, T. Siepmann, and F. Bohlmann, Justus Liebigs Ann. Chem., 661, 1
(1963).
" A. H. Fainberg and S. Winstein, J. Amer. Chem. SOC., 79, 1597, 1602, 1608 (1957).
" (a) G. A. Olah and P. v. R. Schleyer, Eds., Carbonium Ions, Wiley-Interscience, New York, Vol. I,
1968; Vol. 11, 1970; Vol. 111, 1972; Vol. IV, 1973. Other reviews: (b) D. Bethel1 and V. Gold,
Carbonium Ions, Academic Press, London, 1967; (c) N. C. Deno, Prog. Phys. Org. Chem., 2, 129 (1964).
'' G. A. Olah, J. Amer. Chem. SOC., 94, 808 (1972).