Page 234 - Mechanism and Theory in Organic Chemistry
P. 234
Effects of Structure and Solvent 223
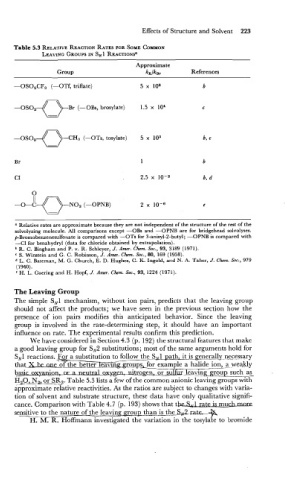
Table 5.3 RELATIVE REACTION RATES FOR SOME COMMON
LEAVING GROUPS IN SN 1 REACTIONS~
Approximate
Group kx/km References
-
-OS0,CF3 (-OTf, triflate)
-CHI (-OTs, tosylate) 5 x lo3 b, c
0
-O-&a-NO, - (-OPNB) 2 x c
Relative rates are approximate because they are not independent of the structure of the rest of the
solvolyzing molecule. All comparisons except -0Bs and -0PNB are for bridgehead solvolyses.
p-Bromobenzenesulfonate is compared with -0Ts for 3-anisyl-2-butyl; -0PNB is compared with
-C1 for benzhydryl (data for chloride obtained by extrapolation).
R. C. Bingham and P. v. R. Schleyer, J. Amer. Chcm. Soc., 93, 3189 (1971).
S. Winstein and G. C. Robinson, J. Amcr. Chcm. Soc., 80, 169 (1958).
L. C. Bateman, M. G. Church, E. D. Hughes, C. K. Ingold, and N. A. Taher, J. Chem. Soc., 979
(1940).
H. L. Goering and H. Hopf, J. Amcr. Chcm. Soc., 93, 1224 (1971).
The Leaving Group
The simple SN1 mechanism, without ion pairs, predicts that the leaving group
should not affect the products; we have seen in the previous section how the
presence of ion pairs modifies this anticipated behavior. Since the leaving
group is involved in the rate-determining step, it should have an important
influence on rate. The experimental results confirm this prediction.
We have considered in Section 4.3 (p. 192) the structural features that make
a good leaving group for SN2 substitutions; most of the same arguments hold for
S,1 reactions. .F&r a substitution to fo!ow the Szl path, it is generally n-esessary
that Xkmeane'ort_he better ~eayjn~~o_up~for example a halide iosa weakly
b-n, ar a mtral ox-nitrsen, or sulfus_leavi_n~ group such as
HaOLN, or SR2. Table 5.3 lists a few of the common anionic leaving groups with
approximate relative reactivities. As the ratios are subject to changes with varia-
tion of solvent and substrate structure, these data have only qualitative signifi-
cance. Comparison with Table 4.7 (p. 193) shows that the2&J rate is mudunme
sensitive to the nature of the leaving group than is the S&ra.te--)&
H. M. R. Hoffmann investigated the variation in the tosylate to bromide