Page 254 - Mechanism and Theory in Organic Chemistry
P. 254
Mechanisms Intermediate Between SN1 and SN2 243
correlation between Y and rate of Reaction 5.20 is excellent over a wide variety of
solvents of varying nucleophilicity; if t-butyl chloride were subject to nucleo-
philic assistance, the Y values would reflect this fact and would not correlate
adamantyl rates for solvents of different nucleophilicity. For very strongly
ionizing solvents such as trifluoroethanol, trifluoroacetic acid, and hexafluoro-2-
propanol, the correlation faikg7 The reason proposed is that in these solvents the
t-butyl substrates are undergoing elimination by attack of solvent on a proton.
The adamantyl systems can neither solvolyze with nucleophilic assistance nor
eliminate, and it has therefore been proposed that 1-adamantyl (or 2-adamantyl,
see below) tosylate be the standard for the Y scale.98 This revised Y scale mea-
sures solvent ionizing power only and does not include any contribution from
solvent nucleophilicity.
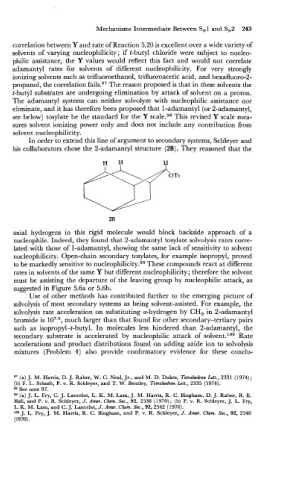
In order to extend this line of argument to secondary systems, Schleyer and
his collaborators chose the 2-adamantyl structure (28). They reasoned that the
axial hydrogens in this rigid molecule would block backside approach of a
nucleophile. Indeed, they found that 2-adamantyl tosylate solvolysis rates corre-
lated with those of 1-adamantyl, showing the same lack of sensitivity to solvent
nucleophilicity. Open-chain secondary tosylates, for example isopropyl, proved
to be markedly sensitive to nucleophilicity.99 These compounds react at different
rates in solvents of the same Y but different nucleophilicity; therefore the solvent
must be assisting the departure of the leaving group by nucleophilic attack, as
suggested in Figure 5.6a or 5.6b.
Use of other methods has contributed further to the emerging picture of
solvolysis of most secondary systems as being solvent-assisted. For example, the
solvolysis rate acceleration on substituting a-hydrogen by CH, in 2-adamantyl
bromide is 107.5, much larger than that found for other secondary-tertiary pairs
such as isopropyl-t-butyl. In molecules less hindered than 2-adamantyl, the
secondary substrate is accelerated by nucleophilic attack of solvent.loO Rate
accelerations and product distributions found on adding azide ion to solvolysis
mixtures (Problem 4) also provide confirmatory evidence for these conclu-
(a) J. M. Harris, D. J. Raber, W. C. Neal, Jr., and M. D. Dukes, Tetrahedron Ltt., 2331 (1974);
(b) F. L. Schadt, P. v. R. Schleyer, and T. W. Bentley, Tetrahedron Ltt., 2335 (1974).
See note 97.
(a) J. L. Fry, C. J. Lancelot, L. K. M. Lam, J. M. Harris, R. C. Bingham, D. J. Raber, R. E.
Hall, and P. v. R. Schleyer, J. Amer. Chem. Soc., 92, 2538 (1970); (b) P. v. R. Schleyer, J. L. Fry,
L. K. M. Lam, and C. J. Lancelot, J. Amer. Chem. Soc., 92, 2542 (1970).
loo J. L. Fry, J. M. Harris, R. C. Bingham, and P. v. R. Schleyer, J. Am. Chem. Soc., 92, 2540
(1970).