Page 276 - Mechanism and Theory in Organic Chemistry
P. 276
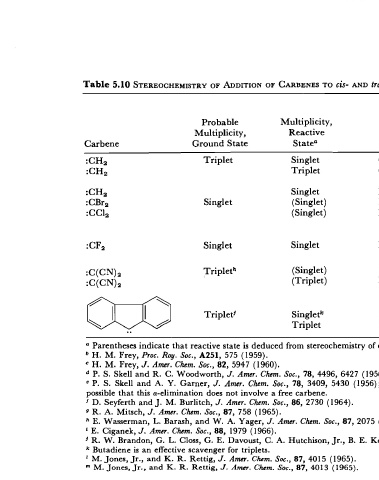
Table 5.10 STEREOCHEMISTRY ADDITION OF CARBENES TO cis- AND tram-2- BUTENE
OF
Products, Percent
cis Addition
Probable Multiplicity,
Multiplicity, Reactive P\ to Refer-
Carbene Ground State Statea Phase Conditions to ences
Triplet Singlet Gas 500 mm pressure
Triplet Gas > 2000 mm; excess
argon
Singlet Liquid
Singlet (Singlet) Liquid a-Elimination
(Singlet) Liquid From 4HgCC1,Br
Singlet Singlet Liquid From F,C/l \
N
Tripleth (Singlet) Liquid Pbre olefin as solvent
(Triplet) Liquid Olefin diluted 100: 1
with c-C6HI2
Triplet' Singletk Liquid Butadiene added
Triplet Liquid Diluted with C6FB
a Parentheses indicate that reactive state is deduced from stereochemistry of olefin addition.
H. M. Frey, Proc. Roy. Soc., A251, 575 (1959).
H. M. Frey, J. Amer. Chcm. Soc., 82, 5947 (1960).
* P. S. Skell and R. C. Woodworth, J. Amer. Chem. Soc., 78, 4496, 6427 (1956).
P. S. Skell and A. Y. Garner, J. Amr. Chcm. Soc., 78, 3409, 5430 (1956); W. v. E. Doering and P. LaFlamme, J. Amer. Chem. Soc., 78, 5447 (1956). It is
possible that this a-elimination does not involve a free carbene.
f D. Seyferth and J. M. Burlitch, J. Amcr. Chcm. Soc., 86, 2730 (1964).
R. A. Mitsch, J. Amer. Chem. Soc., 87, 758 (1965).
E. Wasserman, L. Barash, and W. A. Yager, J. Am. Chem. Soc., 87, 2075 (1965).
' E. Ciganek, J. Amcr. Chem. Soc., 88, 1979 (1966).
j R. W. Brandon, G. L. Closs, G. E. Davoust, C. A. Hutchison, Jr., B. E. Kohler, and R. Silbey, J. Chcm. Phys., 43, 2006 (1965).
Butadiene is an effective scavenger for triplets.
' M. Jones, Jr., and K. R. Rettig, J. Am. Chem. Soc., 87, 4015 (1965).
'" M. Jones, Jr., and K. R. Rettig, J. Amcr. Chem. Soc., 87, 4013 (1965).