Page 272 - Mechanism and Theory in Organic Chemistry
P. 272
Carbenes 261
methvlPnPrnrltu~cd methylems and can occur either inter- or
1
in thetupk&ate+ -traction to yield a
radical pair (lkp&n_5.3) seems a reasonable possibilitYY far the insertion
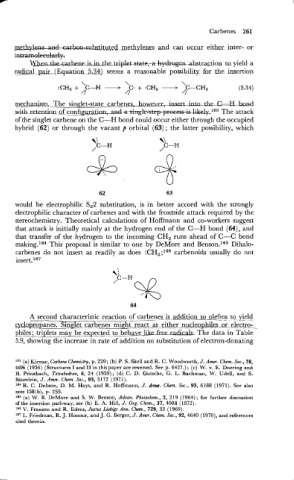
mms-m. The singlet-state carbenes, - Ifft&the C-H
. .
wkhretention of c o n f i ~ u r The attack ~ . ~ ~ ~
~
of the singlet carbene on the C-H bond could occur either through the occupied
hybrid (62) or through the vacant P orbital (63) ; the latter possibility, which
would be electrophilic S,2 substitution, is in better accord with the strongly
electrophilic character of carbenes and with the frontside attack required by the
stereochemistry. Theoretical calculations of Hoffmann and co-workers suggest
that attack is initially mainly at the hydrogen end of the C-H bond (64), and
that transfer of the hydrogen to the incoming CH, runs ahead of C-C bond
making.164 This proposal is similar to one by DeMore and Ben~0n.l~~ Dihalo-
carbenes do not insert as readily as does :CH2;166 carbenoids usually do not
insert.167
A second characteristic reaction of carbenes is addition to olefins to yield
-
~yclopropanes. Singlet carbenes might react as either nucleophiles_sr..e~ectro~~
philes; triplets may be expected to behave like fixcrack& . The data in Table
--
5.9, showing the increase in rate of addition on substitution of electron-donating
183 (a) Kirmse, Carbene Chemistry, p. 220; (b) P. S. Skell and R. C. Woodworth, J. Amer. Chem. Soc., 78,
4496 (1956) (Structures I and I1 in this paper are reversed. See p. 6427.) ; (c) W. v. E. Doering and
H. Prinzbach, Tetrahedron, 6, 24 (1959); (d) C. D. Gutsche, G. L. Bachman, W. Udell, and S.
Bauerlein, J. Amer. Chem. SOC., 93, 5172 (1971).
le4 R. C. Dobson, D. M. Hays, and R. Hoffmann, J. AT. Chem. Soc., 93, 6188 (1971). See also
note 158(b), p. 259.
'e6 (a) W. B. DeMore and S. W. Benson, Aduan. Photochem., 2, 219 (1964); for further discussion
of the insertion pathway, see (b) E. A. Hill, J. Org. Chem., 37, 4008 (1972).
'86 V. Franzen and R. Edens, Justus Liebigs Ann. Chem., 729, 33 (1969).
'8' L. Friedman, R. J. Honour, and J. G. Berger, J. Amer. Chem. SOC., 92,4640 (1970), and references
cited therein.