Page 270 - Mechanism and Theory in Organic Chemistry
P. 270
Carbenes 259
electron paramagnetic resonance and concluded, contrary to Herzberg's original
determination, that the triplet is nonlinear, with a H-C-H angle of about
136°.155 Herzberg reinterpreted the ultraviolet data and found that a nonlinear
structure is consistent with the spectrum.156 The picture that mrges for other
carbenes is that halomethylenes are grod-state singlets with bond a& The
----,
ra~~r=m&hy1enetar~nes, probably also alkyi-
and
methylene~l~~ arezund-state -- triplets-withbo_nd_anxlgs 130-180" and have,
-
---
-
excited singlet states with angles of 100-110". Accurate quantum mechanical
calculations reproduce the experimental rats well for : CH,, : CHF, and
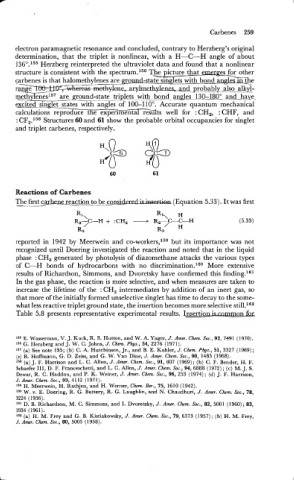
: CF,.158 Structures 60 and 61 show the probable orbital occupancies for singlet
and triplet carbenes, respectively.
Reactions of Carbenes
. .
The f i r - reaction to beconsidered(Equation 5.33). It was first
reported in 1942 by Meenvein and co-w~rkers,~~~ but its importance was not
recognized until Doering investigated the reaction and noted that in the liquid
phase : CH, generated by photolysis of diazomethane attacks the various types
of C-H bonds of hydrocarbons with no discrimination.160 More extensive
results of Richardson, Simmons, and Dvoretsky have confirmed this finding.161
In the gas phase, the reaction is more selective, and when measures are taken to
increase the lifetime of the : CH, intermediates by addition of an inert gas, so
that more of the initially formed unselective singlet has time to decay to the some-
what less reactive triplet ground state, the insertion becomes more selective still.le2
Table 5.8 presents representative experimental results. 1nsertioniu;arnmon far
155 E. Wasserman, V. J. Kuck, R. S. Hutton, and W. A. Yager, J. Amer. Chem. Soc., 92, 7491 (1970).
156 G. Herzberg and J. W. C. Johns, J. Chem. Phys., 54, 2276 (1971).
157 (a) See note 155; (b) C. A. Hutchinson, Jr., and B. E. Kohler, J. Chem. Phys., 51, 3327 (1969);
(c) R. Hoffmann, G. D. Zeiss, and G. W. Van Dine, J. Amer. Chem. Soc., 90, 1485 (1968).
150 (a) J. F. Harrison and L. C. Allen, J. Amer. Chem. Soc., 91, 807 (1969); (b) C. F. Bender, H. F.
Schaefer 111, D. F. Franceschetti, and L. C. Allen, J. Amer. Chem. Soc., 94, 6888 (1972); (c) M. J. S.
Dewar, R. C. Haddon, and P. K. Weiner, J. Amer. Chem. Soc., 96, 253 (1974); (d) J. F. Harrison,
J. Amer. Chem. Soc., 93, 41 12 (1971).
16@ H. Meerwein, H. Rathjen, and H. Werner, Chem. Ber., 75, 1610 (1942).
leO W. V. E. Doering, R. G. Buttery, R. G. Laughlin, and N. Chaudhuri, J. Amer. Chem. Soc., 78,
3224 (1956).
lel D. B. Richardson, M. C. Simmons, and I. Dvoretzky, J. Amer. Chem. Soc., 82, 5001 (1960); 83,
1934 (1961).
lea (a) H. k. Frey and G. B. Kistiakowsky, J. Amer. Chem. Soc., 79, 6373 (1957); (b) H. M. Frey,
J. Amer. Chem. Soc., 80, 5005 (1958).